 Home: PCU
4|2003: Mack Roach III, MD Home: PCU
4|2003: Mack Roach III, MD
 |
|
Mack
Roach III, MD |
| |
| |
Professor, Radiation Oncology and Urology, University of California
San Francisco
Member, University of California San Francisco
Comprehensive Cancer Center
Urologic Oncology Clinical Practice, University of California
San Francisco Comprehensive Cancer Center |
Edited comments by Dr Roach
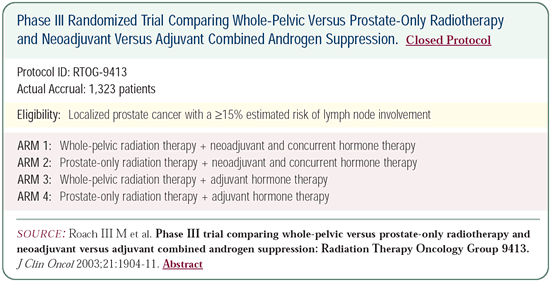
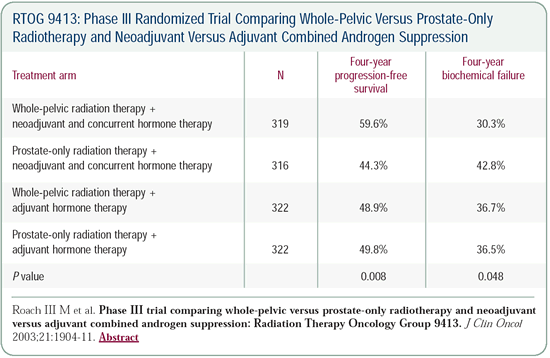
RTOG-9413: Benefits from whole-pelvic radiation
therapy and neoadjuvant combined androgen suppression
We conducted a four-arm randomized trial in 1,300 patients, with
more than 300 patients in each arm. The eligibility criteria included
an estimated risk of lymph node involvement of greater than 15
percent.
Half of the patients received hormone therapy for two months
before and two months during radiation therapy, and the other half
received hormone therapy for four months after they finished radiation
therapy.
Hence, everyone was treated with four months of hormone therapy.
Additionally, half of the patients received radiation therapy
to the pelvic region, including the lymph nodes, while the other
half only received radiation therapy to the prostate. The group
that received hormone therapy two months before and two months
during whole-pelvic radiation therapy had the best outcome.
Clinical implications of RTOG-9413
The clinical implications of RTOG-9413 are huge. These results
explain why trials of radical prostatectomy plus hormone therapy
were negative, while the radiation therapy studies were positive.
If one is going to give patients hormone therapy with radiation,
they should give it before and during radiation therapy.
There are three different groups of patients — those who
don’t need hormone therapy, those who need therapy to improve
local-regional control and those who need long-term hormone therapy
to suppress microscopic disease. In a patient with intermediate
risk, the purpose of hormone therapy is to help control the lymph
nodes.
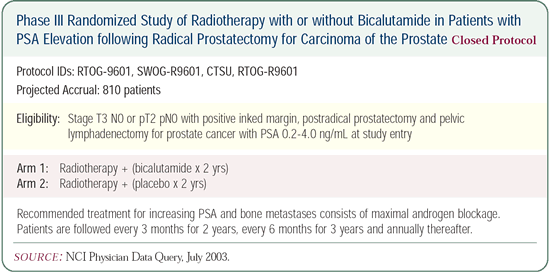
Synergy between hormone therapy and radiation
therapy
There aren’t any preclinical or biologic clues about which
type of hormonal therapy might be most synergistic with radiation
therapy. In RTOG-9413, we used a combined approach with an LHRH
and an antiandrogen. Another question that has not been studied
is: What about an LHRH or an antiandrogen alone? We have a trial
(RTOG-9601) in postoperative patients randomized to bicalutamide
monotherapy with radiation therapy or radiation therapy alone.
The question becomes: What if we had given bicalutamide before
the radiation therapy, and what if we had treated the lymph nodes?
If RTOG-9601 has negative results, we still won’t know the
answers to these questions.
Case Study: 42-year-old man with Gleason 3+4,
T3c prostate cancer
History
In 1997, this man had a PSA of 29.2 ng/mL and was diagnosed with
Gleason 3+3, T2b prostate cancer. He was potent and otherwise healthy
with the exception of hepatitis B. A digital rectal exam revealed
a bulky lesion, mid-gland to the base.
I reviewed his pathology report, and his cancer was upgraded
to a Gleason 3+4. An MRI demonstrated a large volume lesion with
extension to the right seminal vesicle. Using the 1992 staging
system, his tumor was staged as a T3c lesion. He underwent a lymph
node dissection, which was negative.
The patient decided he didn’t want surgery, and I supported
his decision. This was in 1997, so I recommended hormone therapy
and radiation therapy.
We treated him with neoadjuvant hormone therapy for two months
before and two months during radiation therapy. He also received
3D-conformal radiation therapy (7,700 cGy) followed by two years
of the adjuvant hormonal therapy, both were well-tolerated. During
the time he was on hormone therapy, his PSA was undetectable.
He finished his hormone therapy in 2000. His PSA in February
2000 was 0.2 ng/mL, and in May 2000 it was 0.9 ng/mL. We repeated
his PSA in June of 2000, and it was 1.0 ng/mL. The endorectal MRI
and MRS indicated possible abnormal voxels. In July of 2000, he
had an eight-core biopsy, which only revealed radiation effects.
Following the biopsy, his PSAs have remained stable. It’s
been nearly three years since the elevated PSA of 0.9 ng/mL. In
March 2002 and March 2003, his PSA was 0.7 ng/mL, and a repeat
MRI in July 2002 was negative. It has been six years since he began
therapy, and the patient is potent, continent and very happy.
Discussion
This patient was believed to have a T2b lesion, but I was very
concerned that he really had a T3 lesion. It didn’t appear
to be a transition zone tumor, which is one of the things I would
have considered. There are patients with very high PSAs who have
transition zone tumors, and their disease is confined — I
would consider that type of patient, an ideal candidate for a radical
prostatectomy.
Some urologists probably would not have done an MRI and would
have done a radical prostatectomy. After the MRI, some urologists
would still have prefered a radical prostatectomy. They would cite
the data from the Mayo Clinic or the Messing study indicating that
they could give adjuvant hormone therapy for node-positive disease.
However, if there were a type of patient in which urologists would
favor hormone therapy and radiation therapy, this would be the
typical patient, because this is consistent with the Bolla study.
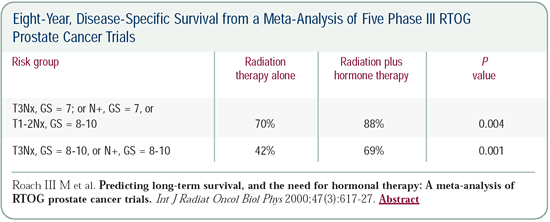
Based on the RTOG meta-analysis, we concluded that patients with
Gleason 7, T3 prostate cancer require long-term hormone therapy
to have the best chance of surviving prostate cancer. This is consistent
with the results from the Bolla study and a newer study, RTOG-9202.
According to the RTOG meta-analysis, the eight-year disease-specific
survival in patients treated this way, but with a lower radiation
dose, was 88 percent. Because we were giving this man a higher
dose of radiotherapy and 3Dconformal radiation therapy, our results
would be better. I would have estimated his eight-year survival
to be over 88 percent and his ten-year survival to be closer to
90 percent.
We just published a study in Urology demonstrating that patients
with pretreatment PSAs greater than 20 ng/mL have a higher overall
mortality from prostate cancer. However, in that study, all patients
were treated with just radiotherapy. In the RTOG meta-analysis,
we did not see a relationship between the pretreatment PSA and
death due to prostate cancer in patients treated with radiation
therapy and hormone therapy. Therefore, hormone therapy will alter
the natural history of prostate cancer.
I have other patients, similar to this man, who have also done
well. I think the use of hormone therapy and radiation therapy
is partially responsible for the decline in prostate cancer mortality
in the United States. I believe that the decline in mortality is
a reflection of the diagnosis and the appropriate aggressive management
of patients with high-risk disease who would have almost been guaranteed
to fail with the conventional therapy of lower-dose radiation alone.
This man would have had a greater than 75 percent chance of recurrence
within five years if he had been treated with low-dose radiation
therapy alone. Now, six years from initial diagnosis, his PSA and
MRI spectroscopy look great and he’s potent.
Today this patient would have been a candidate for RTOG-9902,
comparing long-term hormone therapy and radiation therapy with
or without paclitaxel, estramustine phosphate and etoposide. If
we were going to use implants in such a patient, we would probably
favor high-dose-rate brachytherapy because of the flexibility of
getting needles outside the prostate.
Select publications
|