 Home: PCU 1|2004:Matthew R Smith, MD, PhD Home: PCU 1|2004:Matthew R Smith, MD, PhD

 |
Edited comments by
Matthew R Smith, MD, PhD |
 |
Osteoporotic fractures associated with androgen deprivation therapy
Gonadal steroids regulate bone metabolism in men and women. Androgen deprivation therapy — gonadotropin-releasing hormone (GnRH) agonists and bilateral orchiectomy — dramatically decreases gonadal steroid levels resulting in accelerated bone loss, osteoporosis and fractures. Retrospective studies, the best data available, demonstrate very high fracture rates in men treated with androgen deprivation therapy.
It’s also important to note that osteoporotic fractures are common in older men not being treated with androgen deprivation therapy. Even though the baseline fracture rate in older men is lower than the rate in postmenopausal women, the rates of clinical fractures in men on androgen deprivation therapy are severalfold higher. We may only be observing the tip of the iceberg because the patterns of hormonal therapy utilization have changed dramatically in the past decade, and the late consequences of early androgen deprivation therapy are only beginning to be observed.
Although not particularly common, hip fractures are the most feared and dangerous consequence of osteoporosis; they are associated with high rates of mortality. Vertebral body fractures are more common. They can be asymptomatic, but more commonly they cause pain and the characteristic body habitus changes associated with osteoporosis — loss of height and kyphoscoliosis.
Impact of androgen deprivation therapy on bone metabolism
Both testosterone and estrogen are important for the homeostasis of the skeleton in men; however, estrogen appears to be the dominant regulator of bone metabolism. Estrogen occurs in men as a result of the metabolism of testosterone; therefore, estrogen deficiency is the primary culprit for bone loss related to androgen deprivation therapy. Older men not receiving treatment for prostate cancer have estrogen levels that are in between the levels seen in pre- and postmenopausal women. Estrogen levels in men treated with androgen deprivation therapy are immeasurable — substantially lower than in postmenopausal women.
With a GnRH agonist, testosterone levels fall by more than 95 percent, and estradiol levels also fall by a corresponding amount. This results in an abrupt, dramatic and long-lasting decline in both testosterone and estrogen levels. The rate of bone loss in men treated with androgen deprivation therapy is severalfold higher than in women in early menopause.
Originally, it was believed that the high rate of initial bone loss would be followed by a more gradual loss. However, we’ve observed that these higher rates of bone loss persist even after years of androgen deprivation therapy. This is consistent with the observation that gonadal steroid deficiency is markedly different from the one associated with menopause. The addition of an antiandrogen to medical or surgical castration does not appear to worsen bone loss.
Impact of antiandrogen monotherapy on bone metabolism
Antiandrogen monotherapy does not lower gonadal steroid levels. Through a central feedback mechanism, bicalutamide monotherapy increases the serum concentrations of testosterone and estradiol. Testosterone is blocked by the antiandrogen; therefore, the net effect is somewhat elevated and unopposed estrogen levels, which accounts for the typical side effects of breast tenderness and enlargement. That endocrine profile probably spares the bone in men treated with bicalutamide monotherapy.
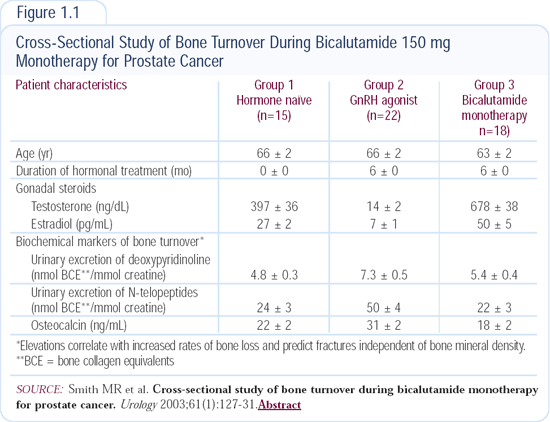
In a cross-sectional study of men treated with bicalutamide monotherapy or a GnRH agonist (Figure 1.1), we’ve evaluated bone turnover markers — a surrogate for bone loss and fractures. Men receiving a GnRH agonist had high rates of bone turnover, and men receiving antiandrogen monotherapy had a bone turnover rate that was consistent with the rate in normal men.
EPC trials of adjuvant bicalutamide monotherapy
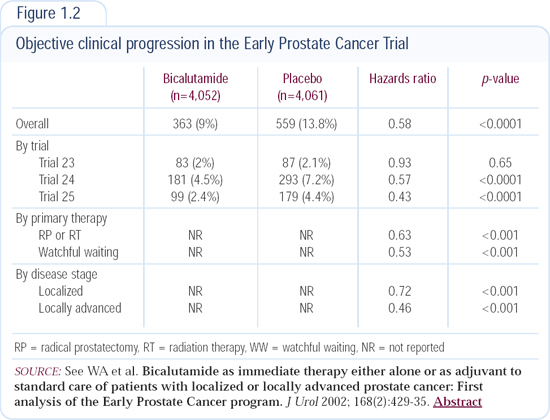
According to results from the adjuvant bicalutamide monotherapy trial (Figure 1.2), a high-risk population will have improved progression rates and survival with sufficient follow-up. In these men with high-risk disease, adjuvant hormonal therapy with castration may also provide a benefit.
If patients were presented with a summary of the quality-of-life, survival and our newer physiologic data, the choice between antiandrogen monotherapy and castration would be clear. They would choose antiandrogen monotherapy. If physicians were asked which they preferred, they would have the same answer, unless they had bone metastases which may result in a modest inferiority in outcome.
A comparison of the side-effect profiles of bicalutamide monotherapy and an LHRH agonist
In terms of the quality of life associated with bicalutamide monotherapy and an LHRH agonist, we have data from completed randomized trials, and the differences are fairly striking. Quality-of-life experts are struck by the magnitude of the differences between those two treatments. For a number of domains — physical capacity, sexual interest, fatigue — bicalutamide monotherapy is statistically better than medical or surgical castration, and the differences are quite large.
For sexual function, the differences are also quite dramatic. Men on androgen deprivation therapy have little or no libido and few, if any, have the ability to maintain an erection. Although bicalutamide monotherapy is not neutral, most men maintain their libido and many, if not most, men who have erectile function are able to maintain it while on therapy.
Medical or surgical castration has a dramatic effect on body composition. After one year, men on androgen deprivation therapy have a 10 percent increase in fat mass and about a three percent loss in lean body mass. The effects of bicalutamide monotherapy on body composition have not been adequately studied.
Two randomized trials are looking at this issue, and the data should be available soon. The fact that bicalutamide monotherapy preserves physical capacity suggests that it also has less adverse effects on physiologic outcomes. Hence, it may cause less fat accumulation and less muscle loss.
The differences in vasomotor symptoms are also striking. Hot flashes occur in the majority of men receiving androgen deprivation therapy, whereas they are nearly nonexistent with bicalutamide monotherapy.
We are also only just beginning to understand the impact of treatment on cognitive function, which is very challenging to measure in aging men because changes occur over time. I believe androgen deprivation therapy does affect cognitive function. In select cases, patients have noticed that their creative, mathematical and writing abilities were altered while on therapy.
Another side effect associated with androgen deprivation therapy is fatigue, which fully impacts on cognitive ability. Men treated with bicalutamide report less fatigue than men receiving androgen deprivation therapy. The superior outcomes with bicalutamide may reflect fewer sleep disturbances related to a lack of vasomotor symptoms, less adverse body composition and less anemia.
Breast effects associated with bicalutamide
The dominant side effects associated with bicalutamide monotherapy are breast tenderness and enlargement, which are probably best managed with either prophylactic breast irradiation, antiestrogens or aromatase inhibitors — all of which have been evaluated for the prevention of the breast effects. A comparative study has been conducted, but the data have yet to be published. My suggestion is to carefully look at the impact of those interventions on other outcomes, like bone and body composition changes.
Some of the advantages associated with bicalutamide monotherapy in the bone relate to the fact that it elevates estrogen levels. Therefore, strategies designed to mitigate the breast tenderness and enlargement may also alter the other effects. For example, an aromatase inhibitor in a man on bicalutamide monotherapy would lower his estrogen levels. This would probably prevent or reduce breast tenderness and enlargement but at the same time contribute to bone loss.
Hormone therapy for PSA relapse
If I personally experienced a PSA relapse, I’d choose antiandrogen monotherapy. There is no evidence that androgen deprivation therapy is better than anti-androgen monotherapy in that setting, and the adverse effects are less with antiandrogen monotherapy.
Antiandrogen monotherapy provides the opportunity to preserve quality of life and effectively treat the disease. For patients in my practice who experience PSA relapse, I point out the known differences between the treatments and the gaps in the data. While the use of bicalutamide 150 mg is considered off label, the use of a GnRH agonist in that setting is also off label.
In the setting of a PSA relapse, I refer to the similarity in survival outcomes in men with nonmetastatic prostate cancer treated with bicalutamide mono-therapy and medical or surgical castration. For men who choose (or I intend to treat with) a GnRH agonist, I’ll briefly discuss the addition of an antiandrogen and the meta-analyses suggesting a modest survival advantage. My firm medical recommendation is that they take it — at least initially — to prevent the potential for flare. Some but not all patients continue it long term.
Select publications
|
| Dr Smith is an Assistant Professor of Medicine at Harvard Medical School in Boston, Massachusetts. |
|