You are here: Home: PCU 1|2005: Adam P Dicker, MD, PhD
| Adam P Dicker, MD, PhD |
EDITED COMMENTS |
 Optimal duration of hormonal therapy for prostate cancer Optimal duration of hormonal therapy for prostate cancer
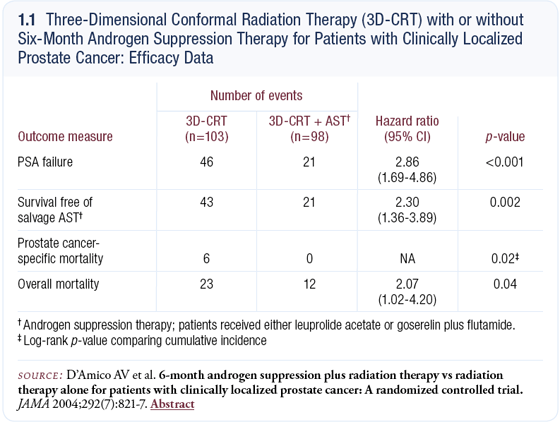
A couple of interesting recent research developments relate to the management of locally advanced prostate cancer. First, D’Amico et al reported results of a clinical trial that randomly assigned patients to receive radiation therapy with or without six months of total androgen suppression (D’Amico 2004; [1.1]).
The group that received hormonal therapy demonstrated a survival advantage, which was surprising because the trial did not accrue a large number of patients. These data raise the question of whether six months of hormone therapy is adequate, or whether we need longer-duration therapy.
Another related article that touches on the duration of hormonal therapy is by a group in Finland who evaluated cognitive function in men — with an average age of 65 — before and after six and twelve months of hormonal therapy (Salminen 2004).
They found a significant decline in memory, time to process information, recall and visuomotor function associated with the decrease in testosterone. Their data do not directly connect hormonal therapy with the decline in psychomotor function, but it is clear to those who treat prostate cancer that long duration therapy — more than one year — impacts patients’ mental acuity.
Clinicians are interested in determining the maximally effective therapy that can be delivered with minimal side effects. When combined with radiation therapy, total androgen suppression may be equivalent to longer duration therapy with an LHRH agonist alone for the treatment of clinically localized prostate cancer.
Impact of endocrine therapy on local tumor control and distant metastases
In radiation oncology, it’s almost a mantra that if we don’t achieve local control, we won’t achieve distant control. This is not only true in prostate cancer; it’s also true in breast cancer. Zietman published an article that basically showed that the metastases rate in prostate cancer is increased when local control is not achieved (Coen 2002). Twenty years ago, everyone treated the whole pelvis with radiation to the nodes, because it was believed that is where prostate cancer spreads; however, that was not based on any evidence. Roach’s Phase III trial, RTOG-9413, comparing whole pelvic to prostate-only radiation therapy and neoadjuvant to adjuvant combined androgen suppression, was the first to demonstrate that large-field radiation therapy with neoadjuvant and concurrent hormonal therapy had a benefit as measured by PSA (Roach 2003).
It appears radiation therapy will probably cure microscopic disease in the nodes, but only when combined with hormonal therapy. I don’t anticipate that radiation therapy alone — at the dose we used, which was limited because of the small bowel — will cure micrometastatic disease. Some people believe hormonal therapy is synergistic with radiation. I’ve seen no evidence of that; rather, it probably has an additive effect.
I would not use the term “radiosensitizer” because hormonal therapy is active by itself, but it certainly augments radiation. I believe hormonal therapy plays a role, but how much of a role it plays locally is unclear. It’s also not clear that the dose used in the Bolla study is sufficient to cure patients. A number of investigators are retrospectively examining their data from patients who received a Bolla-like therapy in various doses during different time periods to determine whether an increase in dose translates to decreased bony metastases and improved survival.
Counseling patients with low-risk disease about local therapy options
Counseling a 65-year-old man with low-grade, low-PSA disease about treatment options is difficult because reasonable data exist for all three major treatment modalities, and the outcome — cancer control and PSA levels — is similar. It becomes a discussion of quality of life and what side effects patients are willing to endure.
For patients 50 years of age or younger, I tell them we don’t know the long-term cancer control rates and I usually steer them toward surgery. The brachytherapy data are now approaching 12 to 15 years of follow-up. I believe we perform brachytherapy well and the outcome data are good, but we have a better handle on outcomes with surgery. Older patients may have other competing risks to their health, whereas younger patients will generally be around for three or four decades, so long-term survival is what counts.
Postimplant CT to re-evaluate results
Based on retrospective data from Richard Stock, we know that to achieve good biochemical control, 90 percent of the prostate should receive approximately 145 Gray with an I-125 prostate implant (Stock 2002). That doesn’t mean the patient won’t be cured if only 85 percent of the prostate is treated, but if a postimplant CT dosimetry showed only 70 percent or less of the prostate was treated, I would have some concerns.
It doesn’t matter whether the CT is performed on the day of the implant or one month later, but it’s better to receive feedback as soon as possible after the implantation. It’s difficult to remember problems you encountered in the operating room, especially if you performed multiple implants on the same day, and it’s important to understand why one patient didn’t receive a good dose.
When the prostate implant results in suboptimal coverage, I tell the patient we’re not happy with what we achieved in the operating room and, assuming I understand why things didn’t go well and the situation can be corrected, my preference is to reimplant the prostate. Others prefer supplemental external beam radiation therapy, but it is difficult to know what dose of radiation therapy to use. I’ve performed 500 to 600 implants in my career, and I’ve only had to reimplant twice. Assuming you didn’t overdose the urethra or the rectum on the first implant, reimplantation shouldn’t cause an increase in complications.
Incorporating chemotherapy into the treatment of prostate cancer
Two trials reported at ASCO 2004 demonstrated a survival advantage in patients with hormone-refractory disease receiving docetaxel-based therapy (Eisenberger 2004; Petrylak 2004). Docetaxel is being extensively evaluated in clinical trials in patients with metastatic disease that is not hormone refractory. Various randomized trials are evaluating hormones with or without chemotherapy in the nonrefractory population. We don’t know if chemotherapy — particularly docetaxel-based chemotherapy — combined with hormones is beneficial in patients with locally advanced disease. Chemotherapy regimens involving taxanes and estramustine have been evaluated, but estramustine has a number of side effects, including deep vein thrombosis. Those studies have been plagued with toxicities and haven’t really moved forward.
Role of chemotherapy in PSA relapse and locally advanced disease
I usually refer patients with PSA relapse and no evidence of skeletal disease to medical oncologists who specialize in prostate diseases. I also encourage them to enroll in clinical trials that evaluate cytostatic therapy or some of the antiandrogen- type drugs. I believe most medical oncologists would be uncomfortable using cytotoxic therapy in a patient who does not have a positive scan. We don’t have any evidence that simply reducing PSA in a patient with nonradiographic metastatic disease has an impact.
Chemotherapy has the potential to harm patients, and we don’t know the optimal duration for chemotherapy. We have preclinical data evaluating the antiangiogenic effects of taxanes (both paclitaxel and docetaxel) in a variety of disease settings. I believe in the next year or two we’ll see chemotherapy being combined more frequently with hormones and radiation therapy in the locally advanced disease setting.
We all agree that a Gleason eight, nine or 10 is locally advanced disease, but we see plenty of tumors with lower Gleason scores and 15 out of 15 positive biopsies. I put those patients in a locally advanced disease category because if they have surgery they will have positive margins, and some will have seminal vesicle and lymph node involvement. It’s a gray area, but patients with a Gleason seven, PSA less than 10 and appropriate performance status may benefit from hormones and chemotherapy.
Select publications
 |
| Dr Dicker is an Associate Professor and Director in the Department of Radiation Oncology’s Division of Experimental Radiation Oncology at the Kimmel Cancer Center, Jefferson Medical College of Thomas Jefferson University in Philadelphia, Pennsylvania. |
|

