 Home: PCU
2|2003: Mitchell Benson, MD
Home: PCU
2|2003: Mitchell Benson, MD
Adjuvant therapy for high-risk patients
Young patients who choose radical prostatectomy do so because
they want to be cured. If they have an adverse pathology report
or a detectable PSA after surgery, it is intellectually inconsistent
to throw our hands up in the air. We have not achieved the desired
result, and we cannot just stop there. That’s not why they
chose radical surgery. They bestow their trust upon us, and I am
very aggressive in treating these patients.
In defense of those not using adjuvant therapy, those of us in
academic urology have not borne our responsibility of enrolling
these patients in clinical trials. However, we cannot wait for all
the statistics before we start altering some of our concepts based
on extrapolation from existing clinical trials.
I believe two years of androgen deprivation should be part of
the standard approach in these patients. If adjuvant hormonal therapy
for breast cancer can improve survival, there's no reason to conclude
that it shouldn’t improve survival in prostate cancer. Some
argue that hormonal therapy only delays progression. But if two
years of hormonal therapy can delay progression for five or six
years, it’s a benefit. The patient on androgen deprivation
is happier than the patient who is progressing. It is a continuum.
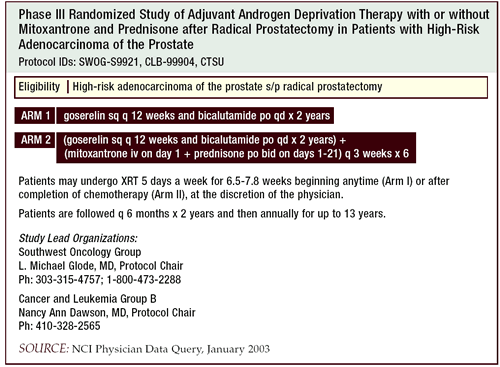
We put many of our high-risk patients on the SWOG Intergroup study
looking at adjuvant hormone therapy alone or with mitoxantrone and
prednisone. This study uses two years of total androgen deprivation
in both arms. Had the study been designed today, perhaps we would
have chosen a docetaxel/estramustine phosphate regimen. The efficacy
data for docetaxel in the metastatic setting seems stronger than
the data for mitoxantrone, and there currently is a clinical trial
trying to prove that.
Adverse pathologic findings to determine use of
adjuvant therapy
Patients with Gleason 7 disease and positive margins or Gleason
8 through 10 disease or seminal vesicle involvement require adjuvant
androgen deprivation. Adjuvant radiation therapy is more controversial.
I'm not sure radiation therapy plays a role for the patient with
negative margins, but any patient with positive margins for whom
I consider adjuvant therapy will also receive radiation therapy
to the bed of the prostate.
Keep in mind, however, that the Gleason scoring system does not
take volume of cancer into account. It is uncommon but not impossible
to have a patient with a Gleason 9 tumor with low-volume cancer.
I don’t treat all of those patients with adjuvant therapy.
It is important to know and talk to your pathologist because not
every pathologist does the same number of slices through a prostate.
If a patient has truly low-volume, high-Gleason disease, depending
upon the clinical circumstances, I might put them on surveillance.
Tolerability of endocrine therapy
If a patient’s fear of dying is great, his tolerance for
toxicity is also great. If his fear of dying is limited, his tolerance
for toxicity is much more limited. Patients on two years of adjuvant
androgen deprivation following radical prostatectomy at age 50 aren’t
happy, but patients with metastatic disease tolerate the side effects
much better.
Some of the complaints in young men are obviously sexual, but
God was kind when he made loss of libido go hand in hand with loss
of ability. It’s one thing to want something you can’t
have, but it’s another thing not to want something you can’t
have. The major complaints have to do with energy changes, loss
of exercise performance and fatigue. The issue is more constitutional
than sexual for many of these patients.
Patients on 150 mg bicalutamide monotherapy maintain more sexual
function, so from the sexual standpoint, patients are very happy.
High-dose bicalutamide may cause some gynecomastia and breast tenderness,
but I haven’t used a great deal of bicalutamide monotherapy
in the adjuvant setting. I use total androgen blockade.
High-dose anti-androgen monotherapy
If you believe total androgen blockade is superior to monotherapy,
it’s hard to believe that high-dose bicalutamide will be superior
to combination therapy because it’s not a total blockade.
But it may be enough. You may not need total blockade in the adjuvant
setting.
Studies have shown that bicalutamide delays progression but the
survival data are not mature. To say that this therapy is truly
efficacious, we really need survival endpoints. I'm not a disbeliever
in the concept of monotherapy, but there is not yet enough information
to make me use it. I’d rather use two years of total blockade
because I just don’t know the survival benefit of bicalutamide
150 mg. Perhaps we'll be able to answer that question with longer
follow-up.
Given the current data showing that bicalutamide delays progression,
I believe it’s very reasonable to present it as an option
to a patient particularly unhappy with the side effects of androgen
deprivation. But we have an obligation to say that we are not sure
this will improve survival. In contrast, we know that total androgen
blockade will delay progression, and there is evidence that it improves
survival.
Continuous versus intermittent androgen deprivation
I believe that the gold standard for treating advanced prostate
cancer remains continuous therapy. We have two open trials comparing
intermittent therapy to continuous therapy. One is in the metastatic
setting and one is for patients with PSA failure following radiation
therapy.
Intermittent therapy only needs to be as good as — not necessarily
better than — continuous therapy for it to become standard
of care. Just as prostate cancer is a heterogeneous disease, patients
themselves are heterogeneous. Intermittent therapy will be worse
in some patients, neutral in others, and there may be some patients
for whom intermittent therapy will be better. Our goal will be to
identify which patients need which treatment.
ProstaScint® scans to guide therapy in patients
with detectable PSA
In theory, the ProstaScint® scan is ideal. The downside is
not the scan itself, but the fact that the antigen to which the
antibody is made is an internal epitope. The prostate specific membrane
antigen, which the antibody aims at, is a transmembrane protein.
Half of the protein sticks out of the cell, and half is inside.
Unfortunately, the antibody used in this imaging study looks at
the portion of the protein inside the cell. It becomes positive
with cell death or cell breakdown antigen exposure. I would expect
the results of imaging studies to be far superior if we aimed at
the external epitope.
The ProstaScint® scan is most helpful in patients post-prostatectomy,
because there is less background noise. It can be useful especially
in those patients at high risk for micrometastatic disease and those
with more aggressive cancers, which have faster growth rates leading
to necrosis of prostate cells and exposure of the internal antigen.
ProstaScint® scans may help dissect out patients with local
disease versus micrometastatic disease in patients with Gleason
7 or 8 through 10 tumors following radical prostatectomy.
We’ve all seen false positives. But, in this country, we
tend not to manage patients by statistics. If there is a one percent
chance of success, we’ll try it because we believe that human
life is so valuable. As a result, ProstaScint® scans get utilized.
They are helpful in some patients, but in many instances, this is
not the sole means for making a decision — unless it’s
so overwhelmingly positive that we have a high reason to believe
it's an accurate result.
Select publications
|