 Home: PCU
2|2003: Richard Stock, MD
Home: PCU
2|2003: Richard Stock, MD
Edited comments by Dr Stock
Real-time ultrasound-guided 3-D brachytherapy
Ultrasound is a superb tool that enables visualization of the
prostate. We developed a real-time approach, which now goes even
further and uses a computer that interacts with the ultrasound images
to give us better seed placement and dose optimization.
There is a learning curve to performing brachytherapy, and radiation
oncologists have not traditionally received much ultrasound training.
Although urologists use ultrasound in their offices, they’re
not using it with the precision that is required to do an implant.
Training also involves how to put the needles in properly and how
to manipulate them, but ultrasound is one of the hardest things
for physicians to master.
Combined-modality trial for men with high-risk
prostate cancer
In 1993, there wasn’t much data about the most important
prognostic features. Initially we treated patients with seed implants
as long as they did not have positive nodes or cancer in the seminal
vesicles on biopsy. We were not giving external beam radiation along
with brachytherapy at that time, and we found that those patients
with high-grade cancers or high PSAs did not do well with just the
seed implants.
Since that was when many of the hormonal therapy trial results
(RTOG trials and early Canadian trials) were coming out, we also
began using hormones as both neoadjuvant and adjuvant therapy. I
felt there was a group of patients who were very high risk —
those with a Gleason score of 8 to 10, a PSA greater than 20 ng/mL
and positive seminal vesicles.
Due to their high-risk features, those patients were more likely,
as we know from the surgical series, to have extracapsular extension.
Therefore, they probably would be better off receiving a combination
of seed implant and external beam radiation.
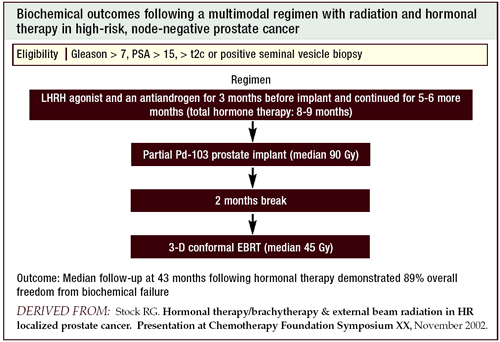
That led us to design a trial in which we gave three modalities
— a higher dose of external beam radiation (59 Gy), a lower
dose of a seed implant and hormonal therapy. It started out as a
Phase I/II trial, and we treated about 40 patients. Initially, we
had superb outcomes, but we started to see increased rectal bleeding.
So we began to lower our external beam radiation therapy dose.
Eventually, there were enough publications demonstrating that this
was a safe way to deliver treatment. We began to give more standard
doses of external beam radiation (45 Gy) and started to include
more patients (i.e., those with Gleason scores of 7 or a PSA greater
than 10 ng/mL). Since we were getting these outcomes in incurable
patients, we decided to include more patients by expanding the inclusion
criteria.
I think that the implant plus external beam gives a much higher
intraprostatic dose than external beam radiation therapy alone.
I’d love to take high-risk patients and compare this regimen
to the best external beam radiation therapy alone, whether it is
IMRT or 3-D conformal.
In more advanced disease, I think this regimen wins out because
the outcomes are better. We even compared our own patients undergoing
radical prostatectomies at Mount Sinai to those treated with this
regimen. There were significant differences in the likelihood of
being biochemically controlled — the rates were more than
twice those with radical prostatectomy — because these patients
are at risk of having extracapsular extension. Therefore, when the
prostate is removed, cancer cells are left behind and there is a
high PSA failure rate.
Adjuvant hormonal therapy
I wrote an editorial in favor of using hormonal therapy with brachytherapy
for the journal Brachytherapy. There are certainly many
who are opposed to the use of hormones, but there is overwhelming
evidence that hormones are absolutely doing something.
The issue is how can you not offer hormonal therapy to high-risk
patients when you treat them with an implant and external beam radiation
or 3D conformal or IMRT? There may not be definitive data indicating
that hormonal therapy works with IMRT or high-dose radiation, but
we know that it improves outcomes with standard radiation.
Decision-making in patients with low-risk prostate
cancer
For patients with low-risk prostate cancer, I let them know about
the options. I tell them that with a seed implant, they are in and
out of the hospital quickly, but they may have irritating symptoms.
If they are the kind of person who couldn’t care less, then
the implant is the way to go. But if they will be bothered by a
sudden strong urge to urinate, then maybe this is not the right
procedure for them. Many urinary symptoms will become worse in the
year following a seed implant.
In men with Gleason 6, low-risk prostate cancer, I tell them that
the available data doesn’t show any significant differences
at this time between brachytherapy and external beam radiation.
I explain what’s involved with the implant, such as coming
to the hospital and going home the same day. I also tell them that
urinary symptoms can occur for a number of months to a year. With
external beam radiation, the patient will have to come in every
day, Monday through Friday, to receive treatment for eight and one-half
weeks, and they may also have some urinary symptoms.
After six or seven years, 30 percent of fully potent men become
impotent because of an implant and close to 40 percent to 50 percent
become impotent because of external beam radiation therapy. I always
tell patients there's no way to really know which treatment is better,
in terms of potency, unless a randomized trial is conducted.
If having the cancer sit in their body even though it is irradiated
will upset the patient, then surgery is probably a better option,
as long as they understand the risks associated with radical prostatectomy.
There are some patients for whom potency is not a real issue, and
they may choose to have surgery.
Brachytherapy for young men with prostate cancer
There is the belief that the follow-up with brachytherapy is short.
But, there is now 10-year outcome data. We have found that most
patients fail within the first three to five years. After five years,
if the patient has a very low PSA, the likelihood of failing is
very small. For me, that is enough follow-up.
The patient’s age makes no difference because there is almost
no incidence of failure after 10 years. In fact, I think sexual
function is much more important for the 45-year-old patient than
for the older patient, and there is no question in my mind that
the potency rate for radical prostatectomy is much lower than with
the seed implant.
“PSA bounce” after brachytherapy
We check the PSA every six months. With brachytherapy, it can
take a long time for the PSA to nadir — four or five years
sometimes. Commonly, there can be a transient elevation in PSA or
a “PSA bounce.”
I wrote a paper for the International Journal of Radiation
Oncology in which we found that PSA increases occur in approximately
40 percent of patients. We looked at the different definitions for
“PSA bounce” that have been cited in literature —
rises in PSA of 0.1 ng/mL or 0.4 ng/mL or anything greater than
a 35 percent rise. Based on these definitions, there were different
incidences of “PSA bounce.” However, none of these different
definitions predicted for failure.
When a patient’s PSA rises, obviously, it could be the first
step in a failure pattern, so the patient must be monitored. On
the other hand, if the patient has low-risk prostate cancer and
the implant was good, it is more likely to be a “PSA bounce”
than a failure since 40 percent of patients have this benign bounce
and in reality, only five to 10 percent will fail.
The most common time for a “PSA bounce” to occur is
around 18 months. Perhaps with failure, the first elevation may
be earlier. Once I’ve seen an elevation, I usually bring the
patient back in three months. Most commonly there is only one elevation,
but there can be two and, rarely, three. Then, the PSA goes back
down.
Younger patients are more likely to have a “PSA bounce.”
So are patients with larger prostates because they probably have
more prostatic epithelium. Therefore, they are more likely to have
inflammation and transient rises.
Forty percent is not an insignificant number, and it is something
that you must counsel patients about. The PSA does go down and,
in the long term, the “PSA bounce” is not a predictor
of failure. We are not sure about its mechanism, so we have to be
very patient and give these men a lot of counseling.
Select publications
|