 Home: PCU
2|2003: Mark S Soloway, MD
Home: PCU
2|2003: Mark S Soloway, MD
CASE 3:
A man with Gleason 7 prostate cancer and a rising PSA one and
one-half years after treatment with androgen deprivation and
external beam radiation |
HISTORY
This 70-year-old man had a clinical T3 prostate cancer with
a Gleason score of 7 and an initial PSA of 25 ng/mL. He received
androgen deprivation and external beam radiation therapy with
continued androgen deprivation for approximately one year.
Three years later, his PSA began to rise and it was clear
that this represented a recurrence.
I discussed different treatment options with the patient,
including LHRH analogue, bilateral scrotal orchiectomy and
bicalutamide 150 mg per day. Of interest, he lives most of
the time in Northern Ireland where bicalutamide is approved
for this indication. He was familiar with it because some
of his friends are being treated with bicalutamide 150 mg.
FOLLOW-UP
The patient has taken bicalutamide 150 mg for a year and
his PSA is 0.8 ng/mL, whereas previously it was approximately
8 ng/mL. He feels quite well, continues to have sexual activity
and the only side effect he’s experienced is gynecomastia,
but it is not disfiguring or incapacitating.
DISCUSSION
The breast enlargement is noticeable, but he’s not
experiencing any breast pain or other side effects. Radiation
therapy to prevent the gynecomastia is a possibility for someone
we know is going to be on therapy for a period of time. There
are not sufficient trials to know how successful it is, but
if it is similar to when we used estrogens many years ago,
it probably would be beneficial. My feeling is that he has
fewer side effects than he would on an LHRH agonist —
he doesn’t have any hot flushes and his cognitive and
sexual functions are excellent. Interestingly, if he were
living here he would not be getting bicalutamide 150 mg because
it’s not available in the United States. |
Trials comparing early versus late hormone therapy
Several important trials have addressed the question of when to
initiate androgen deprivation in the treatment of advanced prostate
cancer. The Medical Research Council (MRC) study compared early
versus late hormone therapy in patients with locally advanced or
asymptomatic metastatic prostate cancer. The study was not perfect.
Whereas we follow patients every three to four months, the study
looked at patients only once a year.
Many patients experienced morbid events before they were started
on hormone therapy, and some even died without ever receiving therapy.
In addition, the “nonmetastatic” group included men
with very high PSAs who almost certainly had metastases. The results
showed that patients treated at diagnosis experienced fewer morbid
events and survived longer than patients randomized to receive androgen
deprivation therapy on progression.
Ed Messing and the Eastern Cooperative Oncology Group conducted
a prospective randomized study of true adjuvant hormonal therapy.
Although a limited study — only 100 patients — the results
indicated that node-positive patients who had radical prostatectomy
followed by immediate hormonal therapy had a lower mortality when
compared to patients who did not receive hormonal therapy until
progression.
The Bolla, or European Radiation Therapy, trial was the largest
study to look at early versus later androgen deprivation therapy
with sufficient follow-up. Patients were high risk with clinically
localized prostate cancer, but it’s likely many had metastases.
They were randomized to receive either radiation therapy alone or
radiation therapy followed by three years of androgen deprivation.
There was a progression-free and an overall survival advantage for
those who received the androgen deprivation therapy.
Oncologic
principles supporting immediate versus delayed hormonal
therapy |
| "Those who advocate limiting the use of ADT until advanced
disease is seen believe that delayed therapy avoids long-term
side effects, reduces cost and utilizes therapy when it is ‘most
needed.’ We will defend the hypothesis that this approach
is inappropriately nihilistic and ignores favorable preclinical
and clinical evidence indicating that ‘early’ ADT
is beneficial. Among the oncologic principles to consider is
the clear demonstration that systemic treatment adjunctive to
local therapy may improve survival. This is clearly the case
in breast cancer where adjuvant hormonal therapy cures more
patients as well as [in] colorectal, gastric cancer and melanoma
where therapies with quite limited activity in advanced disease
extend survival when used adjunctively." |
SOURCE:
Ahmed S, Trump DL. The case for early androgen
deprivation: The data
should not be ignored. Urol Onc 2002;7:77-80. |
Mortality rates in
landmark trials comparing immediate (diagnosis) versus
deferred (progression) hormone treatment for advanced prostate
cancer
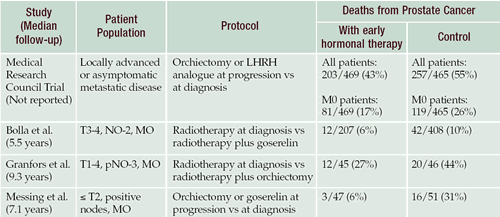
DERIVED FROM:
Bolla M et al. Long-term results with immediate
androgen suppression and external irradiation in patients
with locally advanced prostate cancer (an EORTC study): a
phase III randomised trial. Lancet 2002;360:103-08.
Abstract
Granfors T et al: Combined orchiectomy and external
radiotherapy versus radiotherapy alone for non-metastatic
prostate cancer with or without pelvic lymph node involvement:
A prospective randomized study. J Urol 1998;159(6):2030-4.
Abstract
Messing EM et al. Immediate hormonal therapy compared
with observation after radical prostatectomy and pelvic lymphadenectomy
in men with node-positive prostate cancer. N Engl
J Med 1999;341:1781-8. Abstract
Prostate Cancer Working Party Investigators Group.
Immediate versus deferred treatment for advanced prostatic
cancer: Initial results of the Medical Research Council Trial.
Br J Urol 1997;79(2):235-46. Abstract
|
Early Prostate Cancer Trials: Adjuvant therapy
with bicalutamide 150 mg
The best study to date addressing adjuvant hormone therapy is
the Early Prostate Cancer (EPC) Trials program, which compares adjuvant
bicalutamide 150 mg to placebo. I have no doubt that adjuvant bicalutamide
will delay the time to PSA rise. Castration would have the same
effect, but the side-effect profile is clearly in favor of bicalutamide,
particularly in an otherwise healthy man. Adjuvant LHRH analogues
or orchiectomy may not be appropriate or tolerable for many of our
patients.
We simply have to wait for more events in the EPC trial to know
with certainty whether bicalutamide will favorably impact time-to-progression
and survival. Whether the relatively early data from the EPC should
be presented to all men at this time is an important issue.
The timing of androgen deprivation after local
treatment
The role and timing of androgen deprivation is a critical issue
that urologists encounter on a regular basis. Unfortunately the
available data are not absolutely conclusive due to confounding
factors. Few patients who had radiation therapy initially are candidates
for a salvage radical prostatectomy, so hormonal therapy would be
considered for approximately 95 percent of them. Based on their
age, initial pathology and the time it took PSA to rise, patients
who undergo radical prostatectomy as primary therapy might be candidates
for adjuvant treatment or radiation therapy to the pelvis as salvage
therapy. If local salvage treatment is not the primary option, the
patient will be treated with hormonal therapy, but the question
is when to initiate therapy.
Post-prostatectomy, a PSA rising above 0.4 ng/mL indicates the
presence of prostate cancer, but we don’t know if it would
be better to wait until the PSA level is 3.0 ng/mL or 5.0 ng/mL.
There won’t be any clinical evidence of disease and you might
spare the patient a year of side effects from androgendeprivation
therapy. Following radiation therapy, a PSA above 1.0 ng/mL would
lead to a similar conclusion.
A patient-physician dialogue
In my editorial, Timing of androgen deprivation for prostate
cancer: Benefits versus side effects — A patient-physician
dialogue, I used the phrase “patient-physician dialogue”
because I think it needs to be just that. Clinicians have a variety
of issues they need to discuss with patients. One example is the
implications of a slowly rising PSA. Some patients may not see that
as a significant problem, while others will be upset that their
cancer is not being treated. If it’s going to adversely impact
that patient 24 hours a day, then there may be a major benefit to
treating the patient. But there are quality-of-life implications
to treatment as well — some are minor, but some can be problematic
such as hot flushes or diminished libido. It’s important to
discuss these issues with patients and ensure they understand the
advantages and disadvantages of hormonal therapy, if it is presented
as an option.
My own bias is not to initiate androgen deprivation as adjuvant
therapy in high-risk patients because I like to be evidence-based
in my practice. For the patient with a 50 percent chance of relapsing
over the next two or three years, the available information suggests
adjuvant hormonal therapy will delay clinical progression of their
disease, but I do not know whether it will change their survival.
On the other hand, by treating very early, it may suppress the tumor
sufficiently so that they will never relapse. I do not think it
will promote androgen independence early, as some have suggested.
Those are the unknowns, and we do not have sufficient literature
to compel me to present that information to all patients.
Patient preference in the timing of androgen
deprivation therapy
It was clear from the Miami Patient Town Meeting that patients
have a tremendous interest in being educated about their disease
and participating in decision-making. It was also evident that patients
are very focused on their PSAs. If asked specifically, many would
opt for hormonal therapy relatively early and very few would be
willing to wait until their cancer metastasized. What we are seeing
in clinical scenarios across the country is a high percentage of
patients who receive androgen-deprivation therapy for clinically
metastatic disease, but not as many receiving it for biochemical
relapse.
Watchful waiting as an option for local prostate
cancer
Increasingly, there is more information available to support watchful
waiting as an option for appropriately staged individuals, given
the caveat that the staging is not perfect. Watchful waiting may
be appropriate in patients with a low tumor volume, low PSA and
a low Gleason score—probably less than 7. Biopsies can provide
misinformation, so if an intervention would be curative, a rebiopsy
should be performed.
There was a paper from Johns Hopkins that indicated in appropriate
patients with low-volume disease, untreated patients rarely will
not be curable if they proceed to intervention at a later time.
In this study, almost all of the cases had organ-confined or specimen-confined
disease when they went on to have their prostate removed. This article
provides important data about the safety of watchful waiting in
appropriate cases. That’s an important change that I’ve
adopted in my clinical practice.
Monitoring the watchful waiting patient
In watchful waiting, the patient needs to understand that the
cancer will not go away — that’s not the point of this
option. And he has to understand the biology of prostate cancer
— that it probably takes months to years for a cancer to be
clinically detectable — and it is unlikely that within six
months or one year, his cancer will go from a from a curable to
a noncurable stage.
Monitoring the patient consists of a digital rectal exam, PSA
and biopsies every six months. The biopsies should consist of 10-12
biopsies, which shouldn’t be uncomfortable procedures if performed
with a periprostatic nerve block. No one has studied when to reduce
the frequency of the biopsies, but common sense tells me if the
second set of biopsies has either no cancer or a very low-volume
cancer and the PSA remains unchanged, then one could go to one year,
maybe even longer, between biopsies. The age of the patient would
also be a factor.
Select publications
|