 Home: PCU 2|2004: Robert Dreicer, MD, FACP Home: PCU 2|2004: Robert Dreicer, MD, FACP

 |
Edited comments by
Robert Dreicer, MD, FACP |
 |
Trials of chemotherapy for patients with advanced prostate cancer
At ASCO 2004, results from two large randomized trials in patients with advanced prostate cancer will be presented. One is a trial comparing mitoxantrone and prednisone to single-agent docetaxel administered in two different schedules (weekly or every three weeks). The other is the SWOG trial comparing docetaxel and estramustine to mitoxantrone and prednisone. Both of these studies have the potential to alter practice because they ask survival questions about the role of chemotherapy in patients with androgen-independent metastatic disease.
In a nonprotocol setting, chemotherapy is used for the management of disease-related symptoms. If a survival advantage were reported for a drug combination being compared to the gold standard mitoxantrone and prednisone which doesn't affect survival, a more widespread use of chemotherapy in advanced disease could occur. A survival advantage could also foster and stimulate ongoing clinical trials evaluating the use of chemotherapy in earlier stages of the disease where survival may also be impacted.
Report from the PSA Working Group
The PSA Working Group consisted of prominent urologists, medical oncologists and other researchers who presented an initial report a number of years ago and have now issued new guidelines. In the February 1, 2004 edition of the Journal of Clinical Oncology, the group published a report that defined the problem of a rising PSA after primary treatment (surgery or radiation) and the dilemma in interpreting how to evaluate drugs in that setting. It's a very important publication that provides a basis for conducting trials using similar endpoints so we can begin to interpret outcomes.
The PSA Working Group came up with a definition for biochemical failure, based on the best data published. In patients treated with prostatectomy, it was more straightforward: Biochemical failure was defined by the presence of a PSA of 0.4 ng/mL or greater. In patients failing after radiation therapy, it was more complex. The ASTRO definition was useful, but because it is not directly comparable to the prostatectomy failure definition, ASTRO is trying to revise their guidelines. The PSA Working Group suggested a very minor modification to the ASTRO definition.
Patients with a rising PSA may represent a million or a million and a half men in the United States. It's a very large group in which it is uniquely difficult to conduct trials. These patients don't have symptoms or measurable disease, yet many are obviously worried and anxious. Even worried and motivated patients do not want interventions that have a negative impact on their quality of life.
They prefer rational therapies with some evidence of activity, but we don't know how activity should be measured (e.g. a PSA decline to a certain value maintained for a month, a change in the PSA doubling time). The PSA Working Group discussed different ways of quantifying the impact of a particular therapy and its significance. Prospective trial evidence does not exist to suggest that a 50 percent drop in PSA for two months or a change in the PSA doubling time will alter the natural history of the disease.
One of the recommendations made by the PSA Working Group was that novel drugs should not be tested initially in this subgroup of patients. At times, discordance exists between PSA expression and tumor kinetics. Since the effects of novel drugs on PSA expression are unknown, they should be tested in patients with advanced disease in whom the assessment is more straightforward. On the other hand, the PSA Working Group stated that novel immune-modulating therapies would be appropriate to test in this group of patients. Immune modulation probably won't be as effective in patients with androgen-independent advanced disease, so patients with biochemical relapse are quite amenable to immune-modulating approaches such as vaccine strategies.
Role of hormonal therapy concurrent with salvage radiation therapy in patients with biochemical failure after prostatectomy
Data do not exist to support the use of hormonal therapy concurrently with salvage radiation therapy in patients with biochemical failure. This is clearly an area that needs to be investigated, but it will be very difficult because of the broad variability in the patients who are treated with salvage radiation therapy. The data supporting radiation therapy concurrent with hormonal therapy in patients with locally advanced prostate cancer come from both the RTOG and the EORTC trials, which evaluated two and three years of hormonal therapy. However, six months of hormonal therapy is widely used, even though no data support it.
Hormonal therapy selection in patients with biochemical failure
Based on extrapolation from the ECOG trial in patients with node-positive disease the Bolla and MRC trials early hormonal therapy impacts outcome. I point out to my colleagues who offer antiandrogen monotherapy or other approaches that all of those trials used testicular androgen suppression as their primary modality, not even combined androgen blockade. When I talk to patients I tell them, "We must extrapolate from the trials that utilized testicular androgen suppression." That's what I offer patients, and I acknowledge that I am likely in the minority of physicians.
Combined androgen blockade for at least 30 days is a rational therapeutic approach for a patient with metastatic disease. Beyond that, I try to explain the evidence some favorable, some less favorable about combined androgen blockade, which includes a discussion of the potential cost differential and some minor potential toxicity issues. Then, it's the patient's choice. In the men with biochemical failure, for whom I would at some point suggest therapy, I would use LHRH monotherapy.
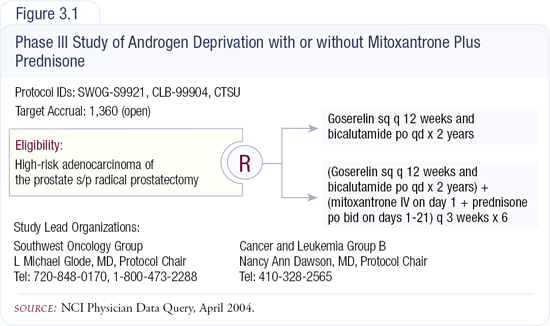
SWOG-S9921 adjuvant trial
SWOG is currently conducting an adjuvant trial in patients with high-risk disease treated with prostatectomy that will compare two years of combined androgen blockade with or without six months of mitoxantrone and prednisone (Figure 3.1). The study was initiated in the late 1990s, and we hope it'll be completed so we have evidence about whether adjuvant therapy truly makes a difference in prostate cancer.
Select Publications
 |
| Dr Dreicer is a Professor of Medicine at the Cleveland Clinic Lerner College of of Genitourinary Medical Oncology and Associate Director of Experimental Therapeutics of Departments of Hematology/Oncology and the Glickman Urological Institute with the Cleveland Foundation in Cleveland, Ohio. |
|