You are here: Home: PCU 2|2005: Anthony V D’Amico, MD, PhD
| Anthony V D’Amico, MD, PhD |
EDITED COMMENTS |
 Survival benefit associated with the combination of hormonal therapy and radiation therapy Survival benefit associated with the combination of hormonal therapy and radiation therapy
The validation that the combination of hormonal therapy and external beam radiation therapy provides a survival benefit compared to radiation therapy alone is an important clinical message.
A number of randomized studies have evaluated this question, particularly in men with localized high-risk disease. “High risk” in this scenario is defined as a Gleason score of seven or higher or a PSA level greater than 10 ng/mL.
The most recent study, published in JAMA in August 2004, demonstrated a 10 percent survival benefit at five years for men who received six months of hormonal therapy in combination with radiation therapy compared to men who received radiation therapy alone (D’Amico 2004; [1.1]).
Hormonal therapy consisted of flutamide with either leuprolide or goserelin. Two questions remain in this scenario: (1) Is combined hormonal blockade necessary? and (2) Are six months of hormonal therapy adequate in patients with Gleason 8, 9 or 10 disease, even if it is T1c or T2?
The studies preceding the trial published in JAMA were RTOG-9202 and the Bolla trial. The Bolla trial — an EORTC study — found that three years of hormonal therapy is better than no hormonal therapy (Bolla 2002). RTOG-9202 found that two years and four months was better than just four months of hormonal therapy. It was not an overall survival benefit but a cancer-specific survival benefit of 3.4 percent at five years (Hanks 2003).
The question still remains whether long-term hormonal therapy is necessary and safe. A European randomized study comparing three years to six months of hormonal therapy should answer the question more definitively. If longterm hormonal therapy truly is better, I suspect that older men (over 70 years of age), in whom occult cardiovascular disease can be prevalent, will benefit least, whereas younger men who don’t have cardiovascular issues may benefit most.
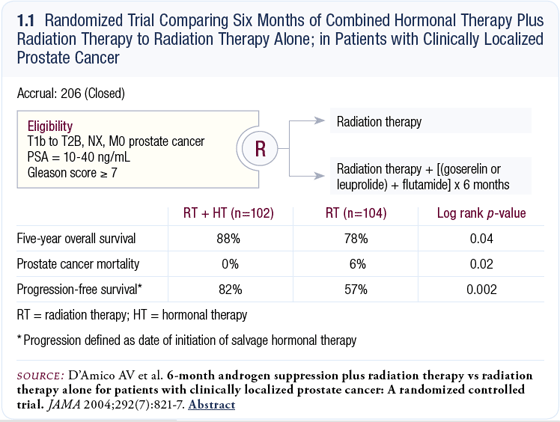
D’Amico trial: Six months of combined hormonal blockade plus radiation therapy versus radiation therapy alone
The study we published in JAMA was a randomized trial in 206 men comparing 3D-conformal external beam radiation therapy (total dose of 70.35 Gray) with or without six months of combined hormonal blockade administered for two months before, two months during and two months after radiation therapy (1.1). In the study, 57 percent of the patients had a PSA that was greater than 10 ng/mL, and 73 percent of the patients had a Gleason score of 7 or higher. This was a study of patients with high-grade cancer. For the most part, patients had T1c disease. More than half the patients had PSA-detected disease, and about 50 percent had T2 or palpable tumors (D’Amico 2004).
The primary endpoint of the trial was progression-free survival. Because the effect of hormonal therapy on cancer-related death was higher than expected, we saw a difference in overall survival, just like the Bolla trial. At five years, progression-free survival was 82 percent for the patients treated with hormonal therapy plus radiation therapy versus 57 percent for those treated with radiation therapy alone. This means the patients treated with radiation therapy alone had a PSA elevation and were on hormonal therapy 25 percent more frequently (D’Amico 2004; [1.1]).
Cancer-specific mortality at five years was zero in the patients treated with hormonal therapy plus radiation therapy versus six percent in the patients treated with radiation therapy alone; overall survival demonstrated a 10 percent difference (88 percent versus 78 percent, respectively). The absolute number of deaths due to prostate cancer was six in the radiation therapy-only arm and zero in the hormonal therapy plus radiation therapy arm (1.1). Out of 206 patients, a six-event difference in prostate cancer deaths was enough to account for a survival difference, mainly because we initially screened patients for cardiovascular disease. The hazard ratio for overall survival was two, which means a two-fold reduction in deaths in the men randomly assigned to combined hormonal therapy plus radiation therapy.
Role of combined hormonal blockade
Off protocol, in patients with high-risk T1c or T2 disease, I use six months of combined hormonal blockade because that is what I used in the study I conducted. In that study, about 27 percent of the patients did not complete the six months of flutamide, mainly because of elevations in their liver function tests (LFTs). They weren’t necessarily having toxicities from the flutamide, but we had a rule: if the LFTs exceeded two times the upper limit of normal, we discontinued the drug for that patient. Despite that, the survival benefit was still seen (D’Amico 2004).
It is an open question whether combined hormonal blockade is really necessary; however, without an answer from a randomized trial, I follow the results from the randomized trial we have. When we designed that trial in 1994, bicalutamide wasn’t available, so flutamide was used. Today, bicalutamide is used because it’s a once-a-day drug and it doesn’t have the same LFT issues.
In patients who have T3 or T4 disease by palpation, I use exactly what the RTOG utilized in their randomized study: two months of neoadjuvant combined hormonal blockade, two months of combined hormonal blockade concurrent with radiation therapy and two years of an LHRH agonist alone (Hanks 2003).
Recent trials comparing two docetaxel-containing regimens to mitoxantrone plus prednisone in patients with hormone-refractory metastatic prostate cancer
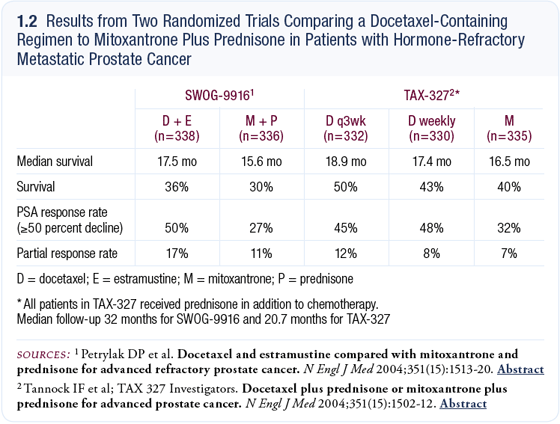
In 2004, results from two trials comparing docetaxel-containing regimens to mitoxantrone plus prednisone in patients with hormone-refractory metastatic prostate cancer were published in the New England Journal of Medicine — one was SWOG-9916 by Dr Dan Petrylak, and the other was TAX-327 by Dr Ian Tannock (1.2). Both studies demonstrated a survival benefit of about two months for the docetaxel-containing regimen (Petrylak 2004; Tannock 2004).
One study combined estramustine with docetaxel (Petrylak 2004), and the other evaluated docetaxel alone (Tannock 2004). Both studies showed a similar prolongation in survival, but because estramustine increased toxicity, it is not considered a necessary part of the regimen. Two dosing regimens for docetaxel were evaluated: every three weeks and weekly. The every three-week regimen appeared to be better (1.2), although the FDA and others are going to validate that in the future. The currently accepted regimen for docetaxel is 75 mg/m2 every three weeks.
Ongoing clinical trials evaluating docetaxel in patients with earlier-stage disease
We are conducting a trial in patients with high-risk disease. Patients are treated with radiation therapy and hormonal therapy with or without docetaxel. The chemotherapy will be administered for two cycles prior to the start of radiation therapy, concurrent with hormonal therapy, and weekly during radiation therapy, so it’s approximately four months of chemotherapy.
Dr Howard Scher is conducting a trial of hormonal therapy with or without docetaxel in patients with rapidly rising PSAs (eg, doubling times less than three to six months) following surgery or radiation therapy. Dr Mario Eisenberger will be conducting a postoperative adjuvant study in men with high-risk features at prostatectomy (ie, seminal vesicle invasion, Gleason score of 8 to 10); patients will receive hormonal therapy and be randomly assigned to docetaxel or no further therapy.
It’s important to select patients carefully for these studies. For example, the vast majority of patients with a PSA failure after local therapy don’t die from prostate cancer. We know now that it’s the rate of rise of the PSA — and not the PSA failure itself — that’s important, so patients whose PSAs are rising quickly are the patients you want to enroll in these studies. The toxicity from chemotherapy occurs up front, and even younger men require some down time during the chemotherapy regimen. They have to be willing to accept an acute decrement in quality of life for a benefit that’s not yet proven.
The study I’m conducting in men with high-risk disease is powered for a hazard ratio of 1.5, whereas the hazard ratio in our study with hormonal therapy was two. With the chemotherapy, we’re hoping to see half the improvement that we saw with hormonal therapy. If we had a 10 percent benefit from hormonal therapy at five years, we’d be happy with a five percent benefit from chemotherapy.
I’m powering the study for survival, pending the validation of a surrogate (eg, progression-free survival). We evaluated progression-free survival in the study of hormonal therapy and radiation therapy because when that trial was designed in 1994, that endpoint was in vogue for hormonal therapy. The benefit from hormonal therapy was more than expected. We also saw a difference in survival; however, no data for chemotherapy in localized prostate cancer in a randomized setting indicate that progression-free survival can be used as an endpoint.
In our trial, prevention of bone metastases is a secondary endpoint that is clinically relevant. If you design a prostate cancer clinical trial powered for survival, you’ll have plenty of power to go back and evaluate progression-free, disease-free and cancer-specific survival. But if you power the trial for an earlier endpoint, you may not have enough power to evaluate the ultimate endpoint. We expect this study will accrue in two years and be reported three to five years later.
Tolerability of docetaxel
Patients whose performance status is good — such as men under 65 years of age — will tolerate docetaxel well. They come in, receive the infusion, go home, have a couple of days with some symptoms and then go back to their routine. Toxic deaths are rare and few patients require hospitalization for complications. Growth factors can be used to bring up counts if need be, and these patients must have their blood counts monitored. This is a new arena, not for medical oncologists, but for the urologists and radiation oncologists who deal with patients with prostate cancer.
Select publications
 |
| Dr D’Amico is Professor and Chair of Genitourinary Radiation Oncology at Brigham and Women’s Hospital, Dana-Farber Cancer Institute, Harvard Medical School, in Boston, Massachusetts. |
|

