You are here: Home: PCU 2|2005: Leonard G Gomella, MD
| Leonard G Gomella, MD |
EDITED COMMENTS |
 Duration and benefit of adjuvant hormone therapy Duration and benefit of adjuvant hormone therapy
We generally recommend two to three years of adjuvant hormonal therapy when treating patients with locally advanced disease, which is based primarily on the data from recent trials. Bolla’s EORTC trial showed superior outcomes in patients who received three years of hormonal therapy, and the RTOG- 9202 showed two years of hormonal therapy resulted in a survival advantage (Bolla 2002; Hanks 2003).
Admittedly, the duration of hormone therapy is controversial. Some institutional studies have suggested as little as six months of hormonal therapy may be beneficial, and that’s possible, but our recommendations rely on the larger multi-institutional trials with thousands of patients.
While the prospective randomized trials all show this approach is effective, particularly in patients with high-risk disease, it’s not advantageous for all patients. Patients with low-risk disease do not appear to benefit from the combination of hormones and radiation, and the side effects may detract from the patient’s quality of life and overall outcome. In the RTOG-9202 trial, an overall survival advantage was seen in patients with high Gleason’s scores; however, in the patients with lower-risk disease, although the combination may enhance PSA control, we don’t see much improvement in survival.
RTOG-9601: Radiation therapy with or without bicalutamide 150 mg
This Phase III randomized study is in patients with PSA relapse following radical prostatectomy. The study is closed to accrual and we are anxiously awaiting the data. This will be one of the most exciting trials to be reported because it will determine whether it’s beneficial to combine hormonal manipulation with radiation therapy in the salvage setting.
RTOG-8531 showed that patients who received radiation and hormones together after radical prostatectomy for unfavorable prostate cancer had a survival advantage over patients who only received radiation therapy (Lawton 2005). I believe RTOG-9601 will also be a positive study because we know the effectiveness of bicalutamide 150 mg in the adjuvant setting (Wirth 2004). Based on the Iverson and See data, it would be a stretch to think the combination would not be more effective than radiation therapy alone.
Bicalutamide 150 mg is approved in over 50 countries around the world; however, it has not received FDA approval in the United States. In Europe, bicalutamide is commonly used as step-up therapy in which patients receive oral agents, such as a 5-alpha reductase inhibitor, with a small dose of bicalutamide. The bicalutamide dose is then increased up to 150 mg before the patient is started on an LHRH analog as their definitive therapy.
Currently at our center, the medical oncologists’ standard salvage regimen for patients whose disease is failing standard androgen ablation is bicalutamide 150 mg. We have seen responses to this regimen last for over a year and a half, so it appears to be reasonable salvage therapy and can be offered to patients. It does appear that a small percentage of men may have an increased cardiac toxicity associated with the drug. The number of men who had adverse cardiac outcomes and the number of increased death rates in the low-risk arms of the EPC studies with bicalutamide 150 mg were low, but noticeable (Iversen 2004; Wirth 2004). These findings may have been statistical aberrations or statistical noise; nonetheless, they need to be further examined.
Although bicalutamide 150 mg is not currently approved for salvage therapy in the United States, I believe it’s appropriate to discuss it with patients for whom it may be suitable, such as those who are sexually active and want to maintain their sexual functioning. Bicalutamide can preserve sexual function, whereas a high percentage of men on an LHRH analog therapy experience significant sexual dysfunction. Quality of life and determining what’s important to the patient have become central issues when considering treatment alternatives in prostate cancer.
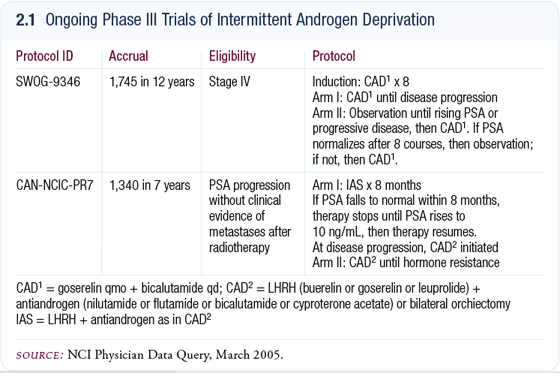
Intermittent hormonal therapy
Intermittent hormonal therapy is not considered the standard of care, but we do use it in select patients. The data on this therapy are conflicting — some preliminary European studies show that it doesn’t adversely affect overall PSA recurrence or survival, whereas other studies report adverse outcomes in prostate cancer progression with intermittent therapy.
One of the challenges is that we are waiting for data on intermittent therapy from the large ECOG trial completed in the United States several years ago. The problem is that this trial evaluated intermittent therapy in patients with high PSA levels and metastatic disease. Most of us believe that for intermittent therapy to work, it will probably be most effective in patients with a low disease burden and minimal PSA elevation.
In fact, we know from the Messing trial that some patients with micrometastatic disease receive hormonal therapy and never have a recurrence (Messing 1999). Certainly some patients who choose to discontinue hormonal therapy will not have disease relapse. This is anecdotal, but I have two young patients who had node-positive, micrometastatic disease with undetectable PSAs postoperatively.
After approximately three years of adjuvant hormonal therapy, they each asked me to take them off of hormonal therapy. They are now approaching almost 10 years since their diagnosis with no evidence of recurrence and they both have normal PSA levels.
What we really need are more studies on intermittent therapy for PSA-only recurrences with low levels (2.1). Because we don’t have the data, we can’t recommend intermittent therapy as a definitive treatment option; however, we can certainly discuss it with patients.
Survival advantage with chemotherapy in metastatic disease
The new data showing a survival advantage with docetaxel-based chemotherapy in patients with hormone-refractory prostate cancer are provocative (Eisenberger 2004; Petrylak 2004). The two large trials reported at ASCO in 2004 have made early chemotherapy a more viable option. The tolerability of docetaxel is also significantly better than the estramustine-based therapies that caused so much toxicity in the 1990s.
At this time, the average patient with a PSA recurrence who has not demonstrated metastatic disease is treated with hormonal therapy front line and, if that fails, another hormone intervention second line. My third line treatment is chemotherapy, because I believe our best opportunity to intercede and have a favorable outcome is in the earliest stages of progression.
For example, we learned that salvage radiation therapy after radical prostatectomy is more effective when used earlier rather than later. We used to initiate salvage therapy when the patient’s PSA reached 4 ng/mL, then 2 ng/mL, then 1.5 ng/mL. Now, for the best outcome, we initiate salvage radiation when the PSA reaches 1 ng/mL. I believe using chemotherapy earlier in the disease is reasonable to consider, although we don’t have any good studies yet to say it should be utilized at the first evidence of PSA recurrence.
We are also seeing an emphasis on a multidisciplinary team approach and consulting with the medical oncologist earlier in the management of prostate cancer. Previously we didn’t have effective chemotherapy regimens to offer patients — nothing demonstrated a statistically significant advantage in large prospective randomized trials until mid-2004, when the two positive docetaxel studies were reported. I believe we will see an intrinsic change in the management of these patients as a result of these data. In addition, other compounds will be available in the next couple of years that may further redefine how patients with PSA recurrence or progressive prostate cancer are managed.
Select publications
 |
| Dr Gomella is the Bernard W Godwin Professor of Prostate Cancer and Chairman of the Department of Urology at Jefferson Medical College and Director of Urologic Oncology at Kimmel Cancer Center in Philadelphia, Pennsylvania. |
|

