
| Tracks 1-16 |
| Track 1 |
Introduction |
| Track 2 |
Influence of PSA velocity on
survival after local therapy |
| Track 3 |
Therapeutic approach to patients
with low-risk disease |
| Track 4 |
Brachytherapy techniques |
| Track 5 |
Medical management of patients
treated with brachytherapy |
| Track 6 |
Erectile dysfunction in patients
treated with brachytherapy |
| Track 7 |
PSA levels after brachytherapy |
| Track 8 |
Clinical algorithm for the
treatment of patients with high-risk
disease |
| Track 9 |
Clinical and research
strategies for patients with
intermediate-risk disease |
|
| Track 10 |
Postprostatectomy radiation
therapy |
| Track 11 |
Management of PSA recurrence |
| Track 12 |
Future role of chemotherapy for
patients with prostate cancer |
| Track 13 |
Key current clinical research
questions in prostate cancer |
| Track 14 |
Brachytherapy with or without
external beam radiation therapy
in patients with intermediate-risk
disease |
| Track 15 |
Case discussion: A 58-year-old
man with Gleason 8 prostate
cancer and seminal vesicle
invasion |
| Track 16 |
Rehabilitation after brachytherapy |
|
|
Select Excerpts from the Interview
 Track 2
Track 2
 DR LOVE: Would you discuss your treatment approach for a patient with
low-risk prostate cancer who has a rapid PSA velocity? DR LOVE: Would you discuss your treatment approach for a patient with
low-risk prostate cancer who has a rapid PSA velocity? |
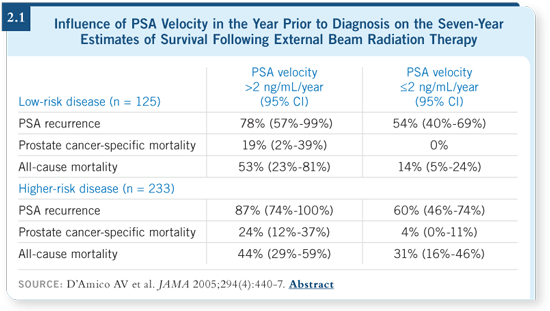
 DR MERRICK: It’s been interesting to evaluate the influence of PSA velocity
on the survival parameters — biochemical, cause specific and overall. Multiple
studies have confirmed that a PSA velocity of greater than 2 ng/mL in the
year prior to diagnosis significantly affects those survival outcomes (D’Amico
2005; [2.1]).
DR MERRICK: It’s been interesting to evaluate the influence of PSA velocity
on the survival parameters — biochemical, cause specific and overall. Multiple
studies have confirmed that a PSA velocity of greater than 2 ng/mL in the
year prior to diagnosis significantly affects those survival outcomes (D’Amico
2005; [2.1]).
 DR LOVE: How have you incorporated those data into your practice?
DR LOVE: How have you incorporated those data into your practice?
 DR MERRICK: Until these data became available, rapid PSA velocity hasn’t been something that we have considered. For patients with low-risk disease,
the cause-specific survival in our brachytherapy series is 99.5 percent at 10
years.
DR MERRICK: Until these data became available, rapid PSA velocity hasn’t been something that we have considered. For patients with low-risk disease,
the cause-specific survival in our brachytherapy series is 99.5 percent at 10
years.
It’s going to be difficult to alter that outcome by looking at any other parameters.
What remains to be determined is how many patients fell into the cohort
of this rapid PSA velocity. We have not yet examined that information.
Patients with low-risk disease are treated with monotherapy. Patients with
high-risk disease receive combined-modality therapy. For patients with intermediate-risk disease, our prospective randomized trials are trying to prove that
we can eliminate the supplemental external beam radiation.
 Track 8
Track 8
 DR LOVE: Would you describe your general algorithm for a patient with
high-risk prostate cancer? DR LOVE: Would you describe your general algorithm for a patient with
high-risk prostate cancer? |
 DR MERRICK: In our program, high-risk disease is defined as two or three
adverse prognostic factors: a clinical stage greater than or equal to T2c, a PSA
greater than 10 ng/mL and a Gleason score greater than or equal to seven. In
our series, all of those patients receive pelvic radiation therapy along with a
palladium boost. A high percentage of those men have also received androgen
deprivation therapy.
DR MERRICK: In our program, high-risk disease is defined as two or three
adverse prognostic factors: a clinical stage greater than or equal to T2c, a PSA
greater than 10 ng/mL and a Gleason score greater than or equal to seven. In
our series, all of those patients receive pelvic radiation therapy along with a
palladium boost. A high percentage of those men have also received androgen
deprivation therapy.
Retrospectively, we have been able to show that the men who received
androgen deprivation therapy have a statistically significant improvement
in eight-year biochemical progression-free survival of approximately eight
percent (Merrick 2005).
We will begin a prospective randomized trial in our Wheeling and Seattle
group later this year, which will randomly assign patients with a Gleason score of seven to nine and a PSA of 10 to 20 ng/mL to 45 Gray of pelvic radiation
therapy and a palladium boost with or without nine months of androgen
deprivation therapy. My expectation is that androgen deprivation therapy will
improve biochemical outcome.
 DR LOVE: Which specific androgen deprivation regimen do you use?
DR LOVE: Which specific androgen deprivation regimen do you use?
 DR MERRICK: We’re strong proponents of total androgen suppression. These
patients will receive nine months of an LHRH agonist, either goserelin or
leuprolide, along with four months of bicalutamide at 50 mg daily.
For all of my patients, I use total androgen suppression for four months. If
the PSA is undetectable at that time, we continue the LHRH alone for the
remainder of the treatment.
DR MERRICK: We’re strong proponents of total androgen suppression. These
patients will receive nine months of an LHRH agonist, either goserelin or
leuprolide, along with four months of bicalutamide at 50 mg daily.
For all of my patients, I use total androgen suppression for four months. If
the PSA is undetectable at that time, we continue the LHRH alone for the
remainder of the treatment.
In the community, what we often see is an LHRH agonist with a short course
of an antiandrogen to block the flare. I have not been a proponent of that
approach. If you look at the RTOG studies, especially the studies that Mack
Roach has conducted, they’ve all used total androgen suppression (Roach
2003; Hanks 2003).
 DR LOVE: It seems that total androgen blockade is not used in the community
as much as it’s used in academia. Is that your impression?
DR LOVE: It seems that total androgen blockade is not used in the community
as much as it’s used in academia. Is that your impression?
 DR MERRICK: It is. I believe the real key with prostate cancer treatment is
to cure the patient up front. We can talk about treatment costs, but we know
that if we treat a patient and cure him with a radical prostatectomy or brachytherapy
or a similar combination, we’re probably talking about a cost of
$15,000 to $40,000.
DR MERRICK: It is. I believe the real key with prostate cancer treatment is
to cure the patient up front. We can talk about treatment costs, but we know
that if we treat a patient and cure him with a radical prostatectomy or brachytherapy
or a similar combination, we’re probably talking about a cost of
$15,000 to $40,000.
Data have been published indicating that once a man experiences biochemical
failure, from failure to death it costs the healthcare industry around $150,000
to $160,000. The curative treatment is always cheap in comparison to treating
patients for the remainder of their lives in a palliative setting.
 DR LOVE: Do you think financial issues are the main reason total androgen
suppression is not used much?
DR LOVE: Do you think financial issues are the main reason total androgen
suppression is not used much?
 DR MERRICK: This regimen does add cost for the patient. Therefore, often
the choice to use it depends on whether the patient can financially afford it.
DR MERRICK: This regimen does add cost for the patient. Therefore, often
the choice to use it depends on whether the patient can financially afford it.
 Track 11
Track 11
 DR LOVE: How do you approach the management of PSA relapse? DR LOVE: How do you approach the management of PSA relapse? |
 DR MERRICK: I’m relatively conservative in managing PSA recurrences. If
we’re going to treat those patients with hormonal therapy, I do not recommend
androgen deprivation therapy until the PSA doubling time becomes less
than 12 months.
DR MERRICK: I’m relatively conservative in managing PSA recurrences. If
we’re going to treat those patients with hormonal therapy, I do not recommend
androgen deprivation therapy until the PSA doubling time becomes less
than 12 months.
Once the PSA doubling time is less than 12 months, we have to seriously
consider androgen deprivation.
The big question then is continuous versus intermittent treatment. I have
always been a proponent of intermittent androgen deprivation therapy because
of the better quality of life associated with it.
 DR LOVE: Would you describe exactly how you use intermittent therapy?
DR LOVE: Would you describe exactly how you use intermittent therapy?
 DR MERRICK: We leave a patient on androgen deprivation therapy for nine
to 12 months. If the PSA becomes undetectable, then we stop the androgen
deprivation therapy until we see the PSA exceed some arbitrary PSA cut point,
such as 10 or 15 ng/mL.
DR MERRICK: We leave a patient on androgen deprivation therapy for nine
to 12 months. If the PSA becomes undetectable, then we stop the androgen
deprivation therapy until we see the PSA exceed some arbitrary PSA cut point,
such as 10 or 15 ng/mL.
 Track 12
Track 12
 DR LOVE: What are your thoughts about the potential future role of
chemotherapy earlier in the natural history of the disease? DR LOVE: What are your thoughts about the potential future role of
chemotherapy earlier in the natural history of the disease? |
 DR MERRICK: This is a reasonable question to evaluate, but it has to be
done in a prospective randomized trial. The studies that Dr Petrylak and
Dr Tannock performed among patients with hormone-refractory disease were
important for a large number of patients. However, it’s also important to
remember that the differences in overall survival were two months (Petrylak
2004; Tannock 2004). These weren’t home runs we were hitting, but they
were small steps, which doesn’t minimize the importance of their work.
However, I do think chemotherapy should, in select cases, be considered
earlier, but it must be done in the setting of prospective randomized trials, not
outside of a protocol.
DR MERRICK: This is a reasonable question to evaluate, but it has to be
done in a prospective randomized trial. The studies that Dr Petrylak and
Dr Tannock performed among patients with hormone-refractory disease were
important for a large number of patients. However, it’s also important to
remember that the differences in overall survival were two months (Petrylak
2004; Tannock 2004). These weren’t home runs we were hitting, but they
were small steps, which doesn’t minimize the importance of their work.
However, I do think chemotherapy should, in select cases, be considered
earlier, but it must be done in the setting of prospective randomized trials, not
outside of a protocol.
Select publications

