
| Tracks 1-14 |
| Track 1 |
Introduction |
| Track 2 |
Surgery versus radiation therapy
for patients with intermediate-risk
prostate cancer |
| Track 3 |
Use of androgen deprivation
therapy for patients with intermediate-risk prostate cancer |
| Track 4 |
Impact of age and body type
on selection of patients for
radical prostatectomy |
| Track 5 |
Use of adjuvant androgen
deprivation for patients with
higher-risk disease |
| Track 6 |
Postprostatectomy treatment
options for patients with
positive margins |
| Track 7 |
PSA recurrence rates after
radical prostatectomy with
positive margins |
| Track 8 |
Postprostatectomy risk of relapse
and treatment options for patients
with seminal vesicle invasion |
|
| Track 9 |
Radiation therapy following
PSA recurrence after
radical prostatectomy |
| Track 10 |
Earlier versus later use of
androgen deprivation therapy for
patients with PSA-only recurrence |
| Track 11 |
Maximal androgen blockade as a
therapeutic option |
| Track 12 |
Treatment options following
PSA rise after primary external
beam therapy |
| Track 13 |
Urologists’ and radiation oncologists’
perception of the benefits
and tolerability of chemotherapy
for prostate cancer |
| Track 14 |
Incorporation of docetaxel
earlier in the treatment of
prostate cancer |
|
|
Select Excerpts from the Interview
 Track 2
Track 2
  DR LOVE: How do you think through the issue of local therapy for young
patients with intermediate-risk disease? DR LOVE: How do you think through the issue of local therapy for young
patients with intermediate-risk disease? |
 DR SOLOWAY: I would generally perform a nerve-sparing prostatectomy
on a young patient with intermediate-risk disease. In my own published data,
no difference was apparent in biochemical recurrence rates among men of all
ages who underwent nerve-sparing versus non-nerve-sparing prostatectomies
(Sofer 2002).
DR SOLOWAY: I would generally perform a nerve-sparing prostatectomy
on a young patient with intermediate-risk disease. In my own published data,
no difference was apparent in biochemical recurrence rates among men of all
ages who underwent nerve-sparing versus non-nerve-sparing prostatectomies
(Sofer 2002).
So unless a finding at the time of surgery indicates that we should take the
nerve bundle because the tumor is present right at the edge of the prostate,
I try to perform a nerve-sparing procedure. More often than not, even in
that situation, if you remove the nerve bundle, you still have a high chance
that no cancer will be present. Leaving only one nerve bundle gives even
a relatively young patient in his late forties a low likelihood of subsequent
normal erections.
Another reason for performing a nerve-sparing procedure is that if the cancer
is indeed localized and you have a positive margin or you monitor the PSA
and intervene at the earliest sign of a biochemical recurrence, you have a
second opportunity with external beam radiation therapy.
 Track 3
Track 3
 DR LOVE: What are your thoughts about using androgen deprivation
therapy for patients with intermediate-risk disease treated with radiation
therapy? DR LOVE: What are your thoughts about using androgen deprivation
therapy for patients with intermediate-risk disease treated with radiation
therapy? |
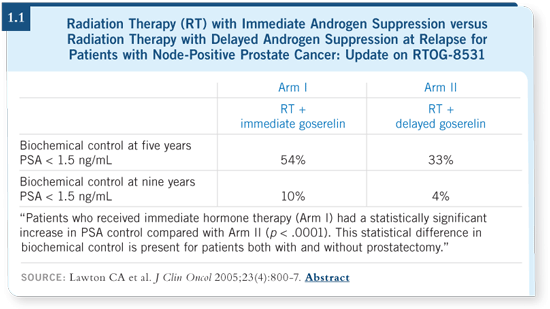
 DR SOLOWAY: The Bolla study and subsequent RTOG studies have made
an impact on practice. Based on these data, one could not disagree with
the use of androgen deprivation in combination with radiation therapy
(Bolla 2002; Hanks 2003; Lawton 2005; [1.1]). The optimal duration of
androgen deprivation is still somewhat unclear. It may be one year, two years
or longer. However, if I were to perform a prostatectomy, I would not add
androgen deprivation.
DR SOLOWAY: The Bolla study and subsequent RTOG studies have made
an impact on practice. Based on these data, one could not disagree with
the use of androgen deprivation in combination with radiation therapy
(Bolla 2002; Hanks 2003; Lawton 2005; [1.1]). The optimal duration of
androgen deprivation is still somewhat unclear. It may be one year, two years
or longer. However, if I were to perform a prostatectomy, I would not add
androgen deprivation.
 Track 10
Track 10
 DR LOVE: How do you approach the issue of androgen deprivation for a
patient with a postprostatectomy rise in PSA? DR LOVE: How do you approach the issue of androgen deprivation for a
patient with a postprostatectomy rise in PSA? |
 DR SOLOWAY: A trend toward earlier androgen deprivation has emerged.
I believe this has been impacted by the Messing study of immediate postprostatectomy
hormonal therapy in patients with positive lymph nodes (Messing
1999). Although it was a small study, it cannot be totally discounted. I believe
a benefit exists with earlier, rather than later, androgen deprivation.
DR SOLOWAY: A trend toward earlier androgen deprivation has emerged.
I believe this has been impacted by the Messing study of immediate postprostatectomy
hormonal therapy in patients with positive lymph nodes (Messing
1999). Although it was a small study, it cannot be totally discounted. I believe
a benefit exists with earlier, rather than later, androgen deprivation.
The PSA screening study in Tyrol, Austria provided diagnoses and initiated
treatment. A reduction in prostate cancer mortality was seen within just a
short number of years, so I believe the impact of hormone therapy did play a
part (Bartsch 2001).
When we initiate androgen deprivation, one big issue is whether to administer
it continuously or to provide the patient with the opportunity for intermittent
therapy. I believe intermittent therapy is a reasonable approach.
I indicate to patients that we have yet to randomize trials indicating the two
regimens are equivalent and that the standard has always been continuous
androgen deprivation. But my own bias, depending on the patient’s age, tolerance
of the androgen deprivation and how long he’s been on therapy, is that it
is reasonable to stop it at some point and monitor the PSA.
 Track 11
Track 11
 DR LOVE: With regard to androgen deprivation, do you use an LHRH
agonist alone or add an antiandrogen? DR LOVE: With regard to androgen deprivation, do you use an LHRH
agonist alone or add an antiandrogen? |
 DR SOLOWAY: If a patient has overt metastatic disease, then I believe
combined androgen deprivation offers some benefit. But in the setting of
rising PSA, in which patients will be on androgen deprivation for many years,
I’m unconvinced that the benefit is substantial enough to add bicalutamide. I
use initial combined therapy for one month and then only the LHRH analog.
DR SOLOWAY: If a patient has overt metastatic disease, then I believe
combined androgen deprivation offers some benefit. But in the setting of
rising PSA, in which patients will be on androgen deprivation for many years,
I’m unconvinced that the benefit is substantial enough to add bicalutamide. I
use initial combined therapy for one month and then only the LHRH analog.
 DR LOVE: Would you present maximal androgen blockade (MAB) therapy to
a patient with a rising PSA as an option if he wanted to pay for it?
DR LOVE: Would you present maximal androgen blockade (MAB) therapy to
a patient with a rising PSA as an option if he wanted to pay for it?
 DR SOLOWAY: I would indicate to the patient that I do believe this approach
provides a slight benefit. I am convinced by the studies and I would follow the
data. I believe Laurence Klotz has best put that information together (Klotz
2001; Prostate Cancer Trialists’ Collaborative Group 2000). I would counsel
a patient that MAB therapy offers some small benefit, and if he accepts the
additional expense, it is reasonable to administer it.
DR SOLOWAY: I would indicate to the patient that I do believe this approach
provides a slight benefit. I am convinced by the studies and I would follow the
data. I believe Laurence Klotz has best put that information together (Klotz
2001; Prostate Cancer Trialists’ Collaborative Group 2000). I would counsel
a patient that MAB therapy offers some small benefit, and if he accepts the
additional expense, it is reasonable to administer it.
 Track 14
Track 14
 DR LOVE: What are your thoughts about trials evaluating docetaxel in the
adjuvant setting? DR LOVE: What are your thoughts about trials evaluating docetaxel in the
adjuvant setting? |
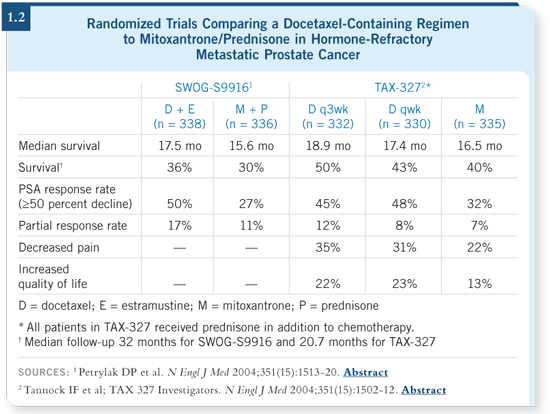
 DR SOLOWAY: A current strategy is to study a drug like docetaxel, which
is currently in trials for high-risk, clinically localized prostate cancer, and
determine whether we can use it to improve the efficacy of our current treatments. Recently, when seeing a patient with high-risk Gleason eight prostate
cancer, a resident asked me about enrolling such patients in trials evaluating
docetaxel as neoadjuvant or adjuvant therapy based on the success of docetaxel
for advanced, metastatic, hormone-refractory disease (Petrylak 2004; Tannock
2004; [1.2]).
DR SOLOWAY: A current strategy is to study a drug like docetaxel, which
is currently in trials for high-risk, clinically localized prostate cancer, and
determine whether we can use it to improve the efficacy of our current treatments. Recently, when seeing a patient with high-risk Gleason eight prostate
cancer, a resident asked me about enrolling such patients in trials evaluating
docetaxel as neoadjuvant or adjuvant therapy based on the success of docetaxel
for advanced, metastatic, hormone-refractory disease (Petrylak 2004; Tannock
2004; [1.2]).
One of the difficulties with docetaxel is that we see relatively few side effects
until about six or eight months after we initiate treatment, and then we start
seeing some significant side effects. When you have a patient who is likely to
live seven to eight years, we should have convincing evidence that we’re going
to affect those years in a positive way before adding those side effects.
 DR LOVE: In the breast cancer model in adjuvant therapy, patients receive
short-term chemotherapy, maybe four or six months, and then long-term
hormone therapy. Prostate cancer in a patient with PSA-only disease is similar
to early breast cancer in that there’s no gross disease. Is it possible that four or
six months of chemotherapy might be worth it in the long run?
DR LOVE: In the breast cancer model in adjuvant therapy, patients receive
short-term chemotherapy, maybe four or six months, and then long-term
hormone therapy. Prostate cancer in a patient with PSA-only disease is similar
to early breast cancer in that there’s no gross disease. Is it possible that four or
six months of chemotherapy might be worth it in the long run?
 DR SOLOWAY: I believe it might be. The question is, do we want to see
the trials first or are we willing to take a leap of faith for our patients with
Gleason eight, nine and 10 disease and say that because it works in metastatic
disease, let’s give the benefit to these patients while we’re awaiting the trial
data? Currently we don’t do that, but I would not argue with someone who
wanted to do so. It may provide a benefit, and it doesn’t have a great deal of
side effects.
DR SOLOWAY: I believe it might be. The question is, do we want to see
the trials first or are we willing to take a leap of faith for our patients with
Gleason eight, nine and 10 disease and say that because it works in metastatic
disease, let’s give the benefit to these patients while we’re awaiting the trial
data? Currently we don’t do that, but I would not argue with someone who
wanted to do so. It may provide a benefit, and it doesn’t have a great deal of
side effects.
Select publications

