|
 Home:
PCU1|2002:
Nancy A Dawson, MD
Home:
PCU1|2002:
Nancy A Dawson, MD
 |
 |
 |
 |
 |
Nancy
A Dawson, MD |
 |
 |
Professor of Medicine
Director, Genitourinary Medical Oncology
University of Maryland Greenbaum Cancer
Center
Vice Chairman, Prostate Committee
Cancer and Leukemia Group B (CALGB) |
|
 |
 |
 |
Edited Comments by Dr Dawson
Prostate Cancer Journal
Club
Pamidronate prevents bone loss associated
with androgen deprivation therapy
Smith MR et al. N Eng J Med 2001;345:948-55.
In this trial, men with nonmetastatic prostate cancer were randomized
to leuprolide plus pamidronate (every 3 months) or leuprolide alone.
All of the patients also received bicalutamide for the first 4 weeks.
In the men treated with leuprolide alone, there was a significant
decrease in bone mineral density. On the other hand, the men treated
with leuprolide plus pamidronate did not have a significant loss
in bone mineral density relative to their baseline.
Although men who are treated with androgen deprivation therapy
have decreased bone mineral density, it is not known whether they
will be at risk for fractures. The emerging data suggests that we
could potentially prevent significant fractures if we avoid a decrease
in bone mineral density. This is an important study supporting the
early use of bisphosphonates in men on androgen deprivation therapy
for prostate cancer. In my practice, I am moving in the direction
of supporting the use of bisphosphonates in all men on androgen
deprivation therapy.
Zoledronic acid prevents skeletal-related
adverse events in men with metastatic hormone refractory prostate
cancer
Saad F et al. American Urological Association Meeting; June
2, 2001.
In a large, multi-institutional, double-blind trial — presented
only in abstract form — men with hormone refractory prostate
cancer that had metastasized to the bone were randomized to zoledronic
acid — a more potent bisphosphonate than pamidronate —
or placebo. Skeletal-related events, such as pathological fractures,
occurred less frequently in the men treated with zoledronic acid.
These results were the basis for the recent FDA approval of zoledronic
acid for the prevention of skeletal-related events in men with bone
metastases from prostate cancer. Another ongoing, randomized, placeboc
o n t rolled trial will evaluate the efficacy of zoledronic acid
in preventing bone metastases in men with PSA-only hormone refractory
prostate cancer. I believe that we should be initiating bisphosphonate
therapy in men with known bone metastases.
Ten-year follow-up of low-risk prostate
cancer treated with brachytherapy
Grimm PD et al. Int J Radiation Oncology Biol Phys 2001;51:31-40
.
The paucity of data on the long-term outcomes associated with
brachytherapy relative to other treatment modalities is frequently
discussed. Finally, the report by Grimm et al provides long-term
follow-up demonstrating that brachytherapy is an effective treatment
for men with lowrisk prostate cancer (PSA <10, Gleason Sum =
2-6, T1-T2b). Of the 125 consecutively treated men, 87% had no evidence
of disease and only 12% had biochemical failure at 10 years. This
is an important paper to consider when counseling men about the
local treatment options and their outcomes.
RTOG 8531: Long-term adjuvant androgen
deprivation following radiation therapy improves survival in men
with Gleason 8-10 prostate cancer
Lawton CAet al. Int J Radiation Oncology Biol Phys 2001;49:937-46.
RTOG 8531 randomized nearly 1,000 men to radiation therapy alone
or radiation therapy plus long-term adjuvant androgen deprivation
with goserelin. Although long-term adjuvant goserelin delayed time
to progression, time to PSA progression and the development of metastases,
there was no improvement in overall survival. In the update to the
trial by Lawton et al, adjuvant goserelin improved the survival
of men with Gleason scores of 8 to 10. In counseling men with high-risk
disease, the improved survival associated with the addition of adjuvant
androgen deprivation to radiation therapy should be discussed.
Bicalutamide as immediate therapy in prostate
cancer reduces the risk of disease progression
Wirth M et al. Urology 2001;58:146-51.
Wirth et al reported results from one of the Early Prostate Cancer
(EPC) trials — an international, multicenter, randomized, placebo-controlled
study that evaluated bicalutamide 150 mg as immediate therapy in
men with localized or locally advanced prostate cancer. This is
the first large trial to investigate whether adjuvant hormonal therapy
improves the outcomes of men with prostate cancer. Men receiving
bicalutamide had a delay in the time to PSA doubling and a significant
reduction in the risk of objective progression. The trial is still
immature and there have not been enough deaths to analyze survival.
If one considers a delay in time to progression an important endpoint,
then this study supports early hormonal therapy. If one is only
concerned about survival, this study suggests that we need to stay
tuned because more information will follow. I hope the delay in
time to pro g ression will lead to an improvement in survival, but
I will need to stay tuned as well.
To decide whether a delay in time to progression is worthwhile,
individual men should be informed of these results and the potential
toxicities associated with bicalutamide. The majority of men treated
with bicalutamide experienced gynecomastia and breast tenderness
or pain. Some patients may decide that these potential side effects
are tolerable in order to delay progression and the onset of metastases.
Other comments
Management of patients with
a rising PSA
In men with a PSA elevation postprostatectomy or postradiation
therapy, there are no clinical trials demonstrating that early hormonal
therapy will improve survival. But the Medical Research Council
(MRC), Bolla and Messing trials — none of which included men
with PSA elevations postprostatecomy/radiation therapy — all
demonstrated that early hormonal therapy was better in terms of
survival. In the MRC trial, early hormonal therapy improved overall
and prostate cancer- free survival for men with asymptomatic locally
advanced prostate cancer. Additionally, early hormonal therapy reduced
the development of spinal cord compressions and fractures . In the
Bolla trial, adjuvant hormonal therapy improved survival in men
with localized prostate cancer who were treated with radiation therapy.
In the Messing trial, adjuvant hormonal therapy improved survival
in men undergoing prostatectomy for node-positive prostate cancer.
Many men watch their PSAs very closely, and they panic when it
is elevated. For those men, preventing a PSA elevation would positively
impact their quality of life. An Intergroup trial is being designed
to compare hormonal therapy with or without chemotherapy in men
with a rising PSA. Presently, the standard of care for a rising
PSA is hormonal therapy.
CHALLENGING CASE 5: 58-year-old man with Gleason
9 prostate cancer

Clinical History
This man had high-risk disease, with bilateral involvement of
the prostate. DRE revealed an enlarged prostate with a nodular left
lobe. PSA was 11 ng/mL. Biopsy resulted in 2/3 and 3/3 positive
cores on the right and left sides, respectively, with a Gleason
score of 9. CT and bone scans were negative.
Key Management Question
What is the optimal systemic therapy for this patient: chemotherapy
and/or hormonal therapy?
Follow-up
The patient was enrolled on a pilot trial evaluating external
beam radiation therapy plus brachytherapy followed by adjuvant chemotherapy
(weekly docetaxel) and 2 years of hormonal therapy (LHRH-agonist).
After 1.5 years, his PSA is undetectable. He is feeling well and
working full time. He has resolving urinary frequency related to
the brachytherapy and impotence due to the hormonal therapy, which
is being treated with sildenafil.
Case Discussion
This man had high-risk disease, with bilateral involvement of
the prostate . Since capsular penetration was likely, I did not
recommend prostatectomy. If he had elected prostatectomy, I would
have encouraged him to enroll in the Intergroup trial which randomizes
men to 2 years of adjuvant hormonal therapy (goserelin/bicalutamide)
plus or minus 6 cycles of chemotherapy ( mitoxantrone / prednisone).
Both groups are randomized to receive 2 years of hormonal therapy.
The Intergroup trial will evaluate the benefit of adding chemotherapy
to hormonal therapy in the adjuvant setting. This patient was also
given the option of enrolling in a University of Maryland pilot
trial to evaluate external beam radiation therapy and brachytherapy
followed by adjuvant chemotherapy (weekly docetaxel) and 2 years
of hormonal therapy (LHRH-agonist). Since the Bolla trial demonstrated
that the addition of hormonal therapy to radiation therapy improved
survival, the non protocol option for this man would have been radiation
therapy in combination with hormonal therapy.
CHALLENGING CASE 6: 80-year-old man with multiple
responses to hormonal therapy

Clinical History
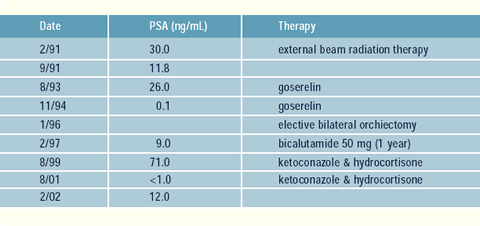
When the patient was 70 years old, DRE revealed a hard nodule
on the right lobe of his prostate (cT3). His PSA was 30 ng/mL, and
his Gleason score was 7. CT and bone scans were negative. He was
treated with external beam radiation therapy. Two years later, he
began a succession of endocrine therapies for PSA elevation (goserelin,
orchiectomy, bicalutamide and ketoconazole/ hydrocortisone), all
of which resulted in PSA responses. Currently, he has progressive
PSA elevation while receiving ketoconazole/hydrcortisone.
Key Clinical Question
What therapeutic strategy should be utilized in an elderly man
with a rising PSA, who responded to prior hormonal therapies?
Follow-up
I have now switched him to DES 1 mg.
Case Discussion
I use the combination of ketoconazole and hydrocortisone as second-line
hormonal therapy after LHRH agonists and bicalutamide. Ketoconazole
inhibits both testicular and adrenal androgenesis; whereas, hydrocortisone
prevents adrenal insufficiency as well as having an antitumor effect.
As second-line hormonal therapy, ketoconazole plus hydrocortisone
has a 60% chance of reducing the PSA. He was started on ketoconazole/hydrocortisone,
and his PSA dropped to less than 1 ng/mL. When that regimen failed,
I utilized DES.
He still remains metastases-free, and his PSA on DES has decreased
to 5. If he progresses again, other therapies to consider would
include PC-SPES, aminoglutethimide, tamoxifen or perhaps an aromatase
inhibitor.
Selected References
Grimm PD et al. 10-year biochemical (prostate-specific antigen)
control of prostate cancer with 125I brachytherapy. Int J
Radiation Biol Phys 2001;51:31-40. Abstract
Lawton CAet al. Updated results of the phase III Radiation
Therapy Oncology Group (RTOG) trial 85-31 evaluating the potential
benefit of androgen suppression following standard radiation therapy
for unfavorable prognosis carcinoma of the prostate. Int
J Radiation Biol Phys 2001;49:937-46. Abstract
Smith MR et al. Pamidronate to prevent bone loss during androgen-deprivation
therapy for prostate cancer. N Engl J Med 2001;345:948-55.
Abstract
Wirth M et al. Bicalutamide (Casodex) 150 mg as immediate therapy
in patients with localized or locally advanced prostate cancer significantly
reduces the risk of disease progression. Urol 2001;58:146-51.
Abstract
|