|
 Home:
PCU1|2002: William A See, MD
Home:
PCU1|2002: William A See, MD
 |
 |
 |
 |
 |
William
A See, MD |
 |
 |
Professor and Chief, Division of Urology
Medical College of Wisconsin
Chairman, Genitourinary Disease Committee
Co-Chairman , Bladder Cancer Subcommittee
Eastern Cooperative Oncology Group |
|
 |
 |
 |
Edited comments by Dr See
The Early Prostate Cancer (EPC) trials
Study design
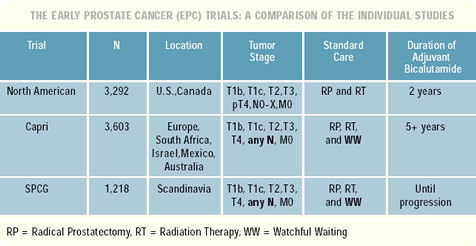
The study was designed for a pooled analysis of 3 individual trials
— the North American trial (Canada and US), the Capri trial
(Europe, South Africa, Central America and Australia) and the SPCG
trial (Scandinavia). Objective disease progression and survival
were the 2 primary endpoints. The 3 trials included men with localized
or locally advanced prostate cancer that was not metastatic.
Cultural variations in the treatment of prostate cancer led to
differences in the 3 trials. In Scandinavia, watchful waiting was
the preferred approach. These watchful waiting patients will provide
meaningful data about early hormonal therapy in men not receiving
primary therapy of curative intent. In North America, watchful waiting
was not routine, and those patients were excluded from the trial.
In contrast to the North American trial, the Capri and SPCG trials
allowed the inclusion of men with node-positive disease. The men
were randomized to immediate bicalutamide 150 mg daily or placebo.
In the North American trial, the average PSA at randomization was
7 ng/mL. In contrast, the average PSA in the SPCG trial was more
than double. These were very different populations in terms of extent
of disease. The North American trial had the best risk population,
which reflects the earlier detection of prostate cancer in this
country.
Progression
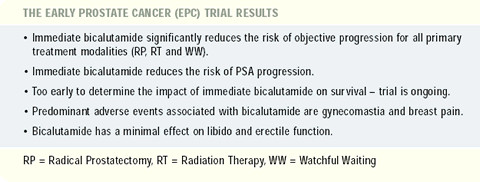
Since the North American trial enrolled patients with the lowest
risk, no demonstrable difference in the risk of objective progression
has emerged for adjuvant bicalutamide. Conversely, in the SPCG and
Capri trials, immediate bicalutamide significantly reduced the risk
of objective progression compared to placebo. When data from the
three trials were pooled, here was a benefit associated with bicalutamide
in all treated patients irrespective of their primary treatment
modality (radical prostatectomy, radiation therapy or watchful waiting),
nodal status, extent of local disease, Gleason score or PSA level
(> 4).
Although we do not yet see a difference in objective progression
for the North American trial, there is a significant difference
in the risk of PSA doubling. If PSA is a predictor of outcome, the
curves for objective progression may eventually separate.
Survival
Approximately 5% of the patients in the trials have died. We have
a long time until we reach the median survival. Therefore, we continue
to follow these patients for objective progression and survival.
In the future, we hope to know the impact of bicalutamide on survival.
Tolerability of bicalutamide
Gynecomastia/breast pain
There appeared to be cultural differences in the tolerance of
drug-related adverse events. In the North American trial, about
17% of the men on bicalutamide withdrew because of an adverse event.
In contrast, only 3% of the men on bicalutamide in the SPCG trial
withdrew due to an adverse event.
The predominant adverse events leading to withdrawal were gynecomastia
and breast pain. Up to 70% of the men treated with bicalutamide
experienced gynecomastia. Since antiandrogens block the pituitary
hypothalamic perception of testosterone levels, there is an increased
production of LH and increased testicular synthesis of testosterone.
The liver and fat, in turn, convert the excess testosterone into
estrogenic compounds. Hence, the estrogenic compounds stimulate
estrogen-sensitive tissue and produce gynecomastia and breast pain.
Breast pain, which is described as sensitivity in the areolar
tissue, is reversible when bicalutamide is discontinued. On the
other hand, gynecomastia persists in 50% to 60% of men when therapy
is discontinued. Breast irradiation may be effective in the prevention
of gynecomastia. Chris Tyrrell is currently studying the efficacy
of single-fraction radiation therapy for the prevention of gynecomastia.
Men were generally more tolerant of breast pain than gynecomastia.
Libido
The SPCG trial evaluated sexual function with a questionnaire.
Relative to placebo, bicalutamide was associated with a small reduction
in sexual interest. This change in libido was less than what would
be expected with an LHRH-agonist.
Bone mineral density
Unlike the LHRH-agonists, preliminary data indicate there are
no changes in bone mineral density associated with the use of bicalutamide
150 mg. The circulating levels of testosterone related to bicalutamide
may protect the bone.
Counseling patients about the EPC data
The proper thing is to have a discussion and let the patient fit
the data into their own personalized risk-benefit ratio. I would
tell a patient, “You have had a primary therapy, either prostatectomy
or radiation, which carries some risk of treatment failure. In your
case, the risk of failure might be X. We have new data suggesting
that adjuvant bicalutamide may reduce the risk of objective progression
and PSA doubling, but we do not know what this means in terms of
overall survival. The majority of men on bicalutamide will develop
gynecomastia and breast pain.”
In the absence of survival data, should we be talking to our patients
about this? Fifteen years ago, we had a similar situation in breast
cancer. Adjuvant tamoxifen, an antiestrogen, was known to reduce
the risk of progression, but at that point, there was no known effect
on survival. Today we know that adjuvant tamoxifen does reduce mortality
significantly. Obviously, there is some uncertainty, but we are
compelled to inform men about the data and allow them to make their
own decision about therapy.
Quality of life considerations in delaying PSA
failure
In my clinical practice, I dread the discussion with men when
their PSA rises after definitive therapy. Since patients can be
devastated emotionally from this experience, there may be value
to delaying a rise in PSA. There may potentially be situations where
survival is not affected, yet the period of illness or disability
may be decreased. The Medical Research Council (MRC) trial, for
example, evaluated early versus delayed hormonal therapy in men
with advanced prostate cancer. Clearly, there was a reduction in
the risk of pathologic fracture and cord - compression with early
hormonal therapy.
Early versus delayed hormonal therapy in men
with prostate cancer
There has been much debate over the use of early versus delayed
hormonal therapy in prostate cancer. Ten years ago, I was in favor
of delayed hormonal therapy. During the last decade, however, evidence
has suggested that earlier intervention may be associated with survival
advantages. Support comes from the Messing trial, which found adjuvant
androgen deprivation to significantly improve survival in men with
node-positive prostate cancer undergoing radical prostatectomy.
The Bolla trial, in men with clinically advanced prostate cancer
undergoing radiation therapy, demonstrated that 3 years of adjuvant
hormonal therapy not only reduced the risk of progression but also
improved survival. These data are prompting a reassessment of our
historic stance on the timing of androgen deprivation. Personally,
I have shifted towards earlier, rather than delayed, hormonal therapy
with the recognition that the optimal timing is unknown.
Selected References
Immediate versus deferred treatment for advanced prostatic
cancer: Initial results of the Medical Research Council Trial. The
Medical Research Council Prostate Cancer Working Party Investigators
Group. Br J Urol 1997;79(2):235-46. Abstract
Bolla M et al. Improved survival in patients with locally advanced
prostate cancer treated with radiotherapy and goserelin. N
Engl J Med 1997;337:295-300. Abstract
McLeod DG et al. Tolerability of bicalutamide (‘Casodex’)
150 mg as immediate or adjuvant therapy in 8113 men with localized
or locally advanced prostate cancer. Proc ASCO 2001;
Abstract
2366.
See WAet al. The bicalutamide early prostate cancer program:
Demography. Urol Oncol 2001;6:43-47. Abstract
Wirth M et al. Bicalutamide (Casodex) 150 mg as immediate therapy
in patients with localized or locally advanced prostate cancer significantly
reduces the risk of disease progression. Urol 2001;58:146-51.
Abstract
Walsh PC et al. A structured debate: Immediate versus deferred
androgen suppression in prostate cancer - evidence for deferred
treatment. J Uro l 2001;166:508-16. Abstract
Wirth M et al. Bicalutamide ("Casodex") 150 mg as
immediate or adjuvant therapy in 8113 men with localized or locally
advanced prostate cancer. Proc ASCO 2001: Abstract
705
|