 Home:
PCU2|2002: Anna
C Ferrari, MD
Home:
PCU2|2002: Anna
C Ferrari, MD
 |
 |
 |
 |
 |
Anna
C Ferrari, MD |
 |
 |
Clinical Associate Professor of
Medicine,
Medical Oncology
Mount Sinai School of Medicine |
|
 |
 |
 |
Edited comments by Dr Ferrari
Lymph node micrometastases in patients with prostate
cancer
In an ongoing trial that will accrue 315 patients, so far out
of 180 cases analyzed, we found the incidence of pathologically
positive pelvic lymph nodes to be about 2 to 3%. In the patients
with pathologically negative lymph nodes, roughly 30% had evidence
of micrometastatic disease by PSA reverse transcription-polymerase
chain reaction (RT-PCR). This indicates the presence of metastatic
cells. I have also analyzed lymph nodes from 60 men without prostate
cancer, and I found no PSA expression by RT-PCR.
PSA recurrence – local or systemic disease?
Patients fail local therapy with radical prostatectomy and radiation
therapy. In our series with Nelson Stone and Richard Stock, prostate
biopsies were taken in patients with high-risk prostate cancer.
The prostate biopsies were negative for recurrence. Therefore, the
disease recurred distantly. However, it may take time to see the
recurrence on a bone scan or a CT scan.
There is no question, in the case of prostate cancer, that PSA
is a relatively good marker, but the more undifferentiated the tumor,
then the more androgenindependent and the less PSA produced. It
is not unusual for a patient to have an almost undetectable PSA
one day, and soon after, they can be riddled with metastases. Does
that tell you the cancer cells multiplied fast? No, the cancer cells
have been there all along. They may just produce less PSA relative
to their parental cells in the prostate. Additionally, some cells
do not produce PSA at all. Although PSA is an excellent tool, it
does not tell us the entire story.
Prostate Cancer Journal Club

Androgen receptor signaling in androgen-refractory
prostate cancer.
Grossmann ME et al. J Natl Cancer Inst 2001;93:1687-97.
This review paper recognizes that the androgen receptor, like
the estrogen receptor in breast cancer, plays a major role in the
control of prostate cancer. To some extent, this has been ignored
in the general prostate cancer literature. Initially, the androgen
receptor uses testosterone and dihydrotestosterone to drive cell
proliferation, differentiation and death. This is why treatment
with hormonal suppression is so successful. Later on when androgen
is suppressed, the androgen receptor, rather than shutting off and
disappearing, remains present and increases its activity several
fold.
The androgen receptor also becomes “promiscuous.”
In the absence of androgens, it will use any other available steroid
hormones (i.e., estrogen, progesterone and glucocorticoids). Additionally,
the androgen receptor can be activated by other growth factors (i.e.,
epidermal growth factor and vascular endothelial growth factor),
which normal cells use to shuffle signals from the environment.
These growth factors can continue to drive prostate cancer cell
survival and proliferation.
Over time, the androgen receptor can develop mutations. These
mutations can turn an androgen receptor antagonist into an agonist.
Many therapies are being developed that actually target the androgen
receptor itself. This paper highlights the significance and implications
of the many different ligands that use the androgen receptor to
drive the progression of the disease. By attacking all the different
signaling pathways and the androgen receptor itself, we may achieve
more. An overexpressed androgen receptor present in prostate cancer
cells, but not normal cells, gives us a window of opportunity whereby
those cells may be targeted more easily. They may be more sensitive,
perhaps, to our interventions. This opens up a whole host of new
therapeutic options that may be developed.
Evidence for the differential expression
of a variant EGF receptor in human prostate cancer.
Olapade-Olaopa EO et al. Br J Cancer 2000;82:185-94.
As prostate cancer progresses, epidermal growth factor receptor
expression in androgen-independent cells is universally and prevalently
expressed. This paper reports on a mutant form of these epidermal
growth factor receptors that are constitutively active. There are
various mechanisms by which receptors can be detrimental. First,
there could be too many of them, and they could be associating when
they should not, like HER2 in breast cancer. In other cases, they
can have a mutation, which keeps them activated and proliferating.
Since there is evidence that the epidermal growth factor receptor
keeps the androgen receptor active, therapies targeting the epidermal
growth factor receptor would be positive. There are two molecules
that are collaborating to sustain androgen-dependent progression.
Therefore, interrupting anywhere along that pathway would be a promising
approach. Obviously, this requires testing in clinical trials.
There are agents that might be available clinically in the next
couple of years. Various companies are developing epidermal growth
factor receptor antagonists and small molecules that target the
epidermal growth factor pathway more downstream. Iressa® is
one of those molecules. There are the antivascular endothelial growth
factor and the COX-2 inhibitors, as well.
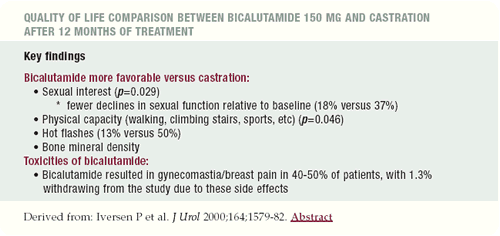
Bicalutamide monotherapy compared with
castration in patients with nonmetastatic locally advanced prostate
cancer: 6.3 years of follow-up.
Iversen P et al. J Urol 2000;164:1579-82.
This paper reports on the long-term results from two randomized
trials that compared bicalutamide 150 mg monotherapy to castration
in 480 patients with locally advanced prostate cancer that was nonmetastatic.
The goal was to determine if there was equivalency between these
two treatments. One offers androgen-sparing therapy, and the other
suppresses testosterone completely.
At the end of 6.3 years, 56% of the patients, who did not receive
any form of local therapy and were only managed with hormonal manipulation,
had died. There was no statistically significant difference in survival
or time to progression between groups. This study essentially demonstrated
that with 6.3 years of follow-up, the two treatments were equivalent.
The results were quite remarkable with respect to quality of life
and tolerability. In terms of sexual interest or libido, there was
a highly statistically significant benefit for the group receiving
bicalutamide monotherapy. There was also a significant difference
in the ability to sustain intercourse.
However, the number of patients who participated in this part
of the study was very small. It is difficult to predict if this
will hold up in a larger patient population, but it makes biologic
sense. There were other significant differences related to quality
of life, particularly physical capacity in terms of well-being,
ability to perform sports, sexual interest, hot flashes and other
activities.
The downsides associated with bicalutamide monotherapy were breast
tenderness and gynecomastia, which are generally not observed with
an LHRH agonist or orchiectomy. The quality of life issues become
very important, particularly in those who will not be able to receive
local therapy. Those patients will receive androgen deprivation
for prolonged periods of time and/or intermittent androgen suppression.
Monotherapy really offers, in my view, a number of quality-of-life
improvements. Not just for men in their fifties or sixties; I have
patients in their eighties who switch to bicalutamide monotherapy,
once they learn about it.
Challenging Case 2:
61-year-old professional dancer with Gleason 8,
hormone-independent, prostate cancer

Clinical history
In 1991, this 61-year-old black male, professional dancer was
diagnosed with Gleason 8, prostate adenocarcinoma. He was treated
with external beam radiation therapy. Subsequently, he had a rising
PSA and was treated with an LHRH analog and an antiandrogen for
four years. Then, he developed metastases to the iliac bone with
some pain. He was referred for evaluation and further treatment.
His main concern was that chemotherapy would interfere with his
quality of life and ability to perform.
Key management question
What treatment options are available to this man?
Follow-up
He had a second hormonal manipulation, antiandrogen withdrawal,
which was not effective. Then, he had a third hormonal manipulation,
another antiandrogen, which worked for a brief period of time. His
PSA continued to rise to 56 ng/mL.
At that point in 1995, he reluctantly started chemotherapy. He
entered a clinical trial with paclitaxel and estramustine. Since
he was one of the first patients on the trial, he got a very low
dose and, therefore, did not respond well. Then, his doses were
increased, and he had a 50% PSA response. Through all of this, he
continued to perform and dance. After 1998, his disease continued
to progress, and he was switched to mitoxantroneprednisone. He did
not have a response in his PSA, but he did have a symptomatic response
in his pelvic pain.
He then went off therapy completely for a number of years. His
PSA ranged anywhere between 170 to 200 ng/mL. When his PSA jumped
to 250 ng/mL, he enrolled on an arsenic trioxide phase II trial.
He had a 50% PSA response, completed the regimen and then went on
to progress. Up until then (February 2000), he continued his normal
activity — besides being a dancer, he had a full-time job.
After that, he retired and was off all therapy. Finally, he developed
disseminated intravascular coagulation from bone metastases, had
a subdural hematoma and died.
Case discussion
Since this patient had received extensive pelvic radiation for
his prostate cancer, he could not receive further radiation to the
pelvic bone for his pain. After a second and third hormonal manipulation,
he reluctantly decided to start chemotherapy. He had a preconceived
notion that he would be nauseous, vomiting, in the hospital and
not able to lead his life.
However, he was fully active, lived by himself, never needed home
assistance, never had any type of medical support and was only admitted
once to the hospital. From 1995 to 2002 — for seven years,
he lived a full life on chemotherapy, but he adapted to the idea
that he had a chronic disease. He lived with androgen-independent
metastatic prostate cancer for seven years with various chemotherapy
manipulations. Once he lost the fear of chemotherapy and realized
that his quality of life would not be impaired, he was willing to
take any therapy. This illustrates how much we have advanced in
the management of metastatic prostate cancer. It is not unusual
to see this kind of course in patients like this.
Once patients enter the phase of androgen-independent, hormone-refractory
prostate cancer, they can compare it to having diabetes and requiring
insulin. Sometimes, they can remain on the same dose for a long
time, but sometimes it does not work. They may have to change the
type of insulin, the insulin schedule and/or the type of agent.
Oncologists and patients must be willing to change therapies. One
has to move on in the process, not let the process dominate. If
the PSA rises with one regimen, it does not mean it will not respond
to the next.
Select publications
|