 Home:
PCU2|2002: Judd
W Moul, MD
Home:
PCU2|2002: Judd
W Moul, MD
 |
 |
 |
 |
 |
Judd
W Moul, MD |
 |
 |
Professor of Surgery,
Uniformed Services University of the Health
Sciences
Attending, Urologic Oncology
Walter Reed Army Medical Center
Director,
Center for Prostate Disease Research
Department of Defense |
|
 |
 |
 |
Edited comments by Dr Moul
Evaluation and management of a PSA recurrence
Proper counseling is essential, and I block an hour off my schedule
to spend the time going through the options.
For a prostatectomy patient, the first option to consider is salvage
radiation therapy to the prostatic bed if we think the disease is
localized. I may do a ProstaScint scan to determine if there is
occult systemic disease in the lymph nodes, either in the pelvis
or the retroperitoneum. If the man has a positive ProstaScint scan,
showing pelvic or retroperitoneal adenopathy, I will not offer radiation
therapy, because he probably has systemic disease.
I believe about 70% of the patients with a PSA recurrence have
systemic occult prostate cancer. They will either be on watchful
waiting to monitor how quickly their PSA is rising or go on systemic
therapy if they are very nervous about the PSA recurrence. If they
decide to go on systemic therapy, the mainstay is hormonal therapy.
We counsel them about traditional hormonal therapy (i.e., an LHRH
agonist or complete androgen blockade) or nontraditional hormonal
therapy. Because of some of the newer data, I think the current
favorite approach in terms of nontraditional therapy would be bicalutamide
150 mg.
Natural history of a PSA recurrence following
radical prostatectomy
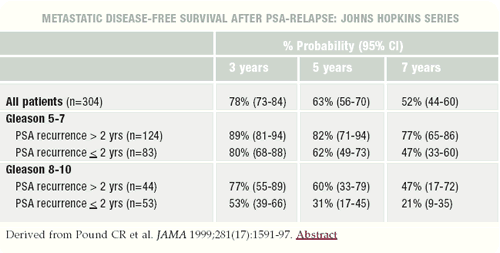
Charles Pound, MD, from the Johns Hopkins group, published in
JAMA in 1999 the best data on the natural history of a PSA recurrence
postprostatectomy. Their data suggest that once the PSA rises to
0.2 ng/mL, it takes about eight years before patients develop a
positive bone scan if they receive no other therapy. After the patients
develop a positive bone scan and receive hormonal therapy, the median
survival is another five years.
I use the Pound paper extensively in counseling patients. We have
the figures and tables from the paper laminated and available in
our exam room. The good news is — that approach appears to
result in about a 13-year survival. The bad news is — that
is not very reassuring for individual patients, particularly younger
ones. Using that article, many more men select early treatment,
rather than watchful waiting, when they have those specific time
lines to apply to their individual life.
Three prognostic factors emerged from the Pound paper —
a PSA doubling time less than 10 months, a pathologic Gleason score
of 8-10 and a PSA recurrence within the first two years. Those were
all poor prognostic factors that moved the eight-year time to a
positive bone scan down to the four- or five-year range. Clearly,
more of those men will take active treatment.
Timing of hormonal therapy
In a man with a rising PSA after surgery or radiation, I take
into account his age, overall health status and psychological makeup.
I try to gauge his feelings about a rising PSA and his understanding
of the implications of a rising PSA. Then, we talk about treatment
options.
In general, my philosophy is to favor early hormonal therapy in
many of these men — particularly the younger, healthier men.
If we follow these men expectantly and they develop a positive bone
scan eight years later, most will still be quite healthy, vigorous
and in good shape. Since we do not have a cure for metastatic prostate
cancer, a positive bone scan essentially means a death sentence
from prostate cancer. Traditional hormonal therapy works well, but
typically patients with stage D2 disease have about a five-year
survival. So, I am making an assumption that using earlier treatment
to change the natural history is reasonable. If we increase the
eight-year time to metastases, hopefully we can extend the time
to death to 15, 17 or 19 years. Then, we can be more assured that
the man will die of some other cause.
Center for Prostate Disease Research (CPDR) database
In the next couple of years, we will have important data on this
question from the Center for Prostate Disease Research (CPDR) database,
a Department of Defense-funded initiative that includes nine major
military medical centers throughout the United States collecting
data on prostate cancer patients. The CPDR database has enrolled
about 15,000 patients.
The database will give further insight into the controversy over
early versus delayed hormonal therapy for PSA recurrence. At the
American Urological Association 2002 Annual Meeting, CPDR presented
data suggesting that early hormonal therapy delays the time to a
recurrent rise in PSA. In 187 men with a postprostatectomy PSA recurrence,
traditional hormonal therapy was started before the PSA reached
a level of 3.0 ng/mL. These men in the database were followed for
a mean of five years. On average, it took about ten and one-half
years for the men to experience a further rise in PSA. So, hormonal
therapy allows an average of 10-11 years before the PSA starts to
rise again.
We do not know if that will change the natural history of a PSA
recurrence as reported in the Pound paper. Will hormonal therapy
change the time to a positive bone scan from eight years to a longer
period? Beyond the time to a positive bone scan, what about the
ultimate survival? We are not able to obtain that information yet.
Traditional versus nontraditional hormonal therapy
In a man with a rising PSA for whom I am considering hormonal
therapy, first I counsel him about traditional hormonal therapy
options — orchiectomy, LHRH agonists alone or in combination
with an oral antiandrogen. Even though it is debatable, some men
choose traditional hormonal therapy because they believe it offers
the best chance of disease control and survival. Other men are reluctant
to take traditional hormonal therapy because of the side effects
— loss of libido, loss of sexual function, hot flashes, weight
gain and osteoporosis. Those side effects are becoming more relevant
in our younger and healthier men. But, it is important to counsel
them about those options.
After discussing the traditional hormonal therapies, I counsel
them about nontraditional hormonal therapy approaches — intermittent
hormonal therapy, oral combination therapy (low-dose bicalutamide
or flutamide with finasteride) and antiandrogen monotherapy. Recently
in practice, I think we are seeing — as a result of the Early
Prostate Cancer (EPC) trial comparing adjuvant bicalutamide 150
mg to placebo — more willingness to use that particular therapy
for PSA recurrence.
An advantage to bicalutamide 150 mg is that, in nonmetastatic
prostate cancer, it is equivalent to traditional hormonal therapy.
The disadvantages include the fact that we do not know about its
efficacy beyond six years relative to traditional hormonal therapy.
In a man with a rising PSA, there is uncertainty as to whether bicalutamide
150 mg is as effective as an LHRH agonist in the long term. But,
there are fewer side effects associated with bicalutamide 150 mg
than traditional hormonal therapy. Bicalutamide 150 mg does not
cause weight gain, hot flashes or a loss of muscle mass. Patients
on bicalutamide 150 mg are able to maintain their libido and sexual
function, particularly if they had a nerve-sparing prostatectomy.
The one unique downside to antiandrogen monotherapy is gynecomastia
and breast tenderness. In my own practice when I offer antiandrogen
monotherapy, I strongly encourage patients to seek a radiation oncology
consultation for prophylactic, low-dose breast irradiation to prevent
the breast side effects.
Since the results from the Early Prostate Cancer trial were presented,
we have become more comfortable offering bicalutamide 150 mg to
patients with a PSA recurrence. I have personally treated about
three or four men in the last several months who have elected bicalutamide
150 mg for a PSA recurrence.
Adjuvant hormonal therapy
When combining all 8,000 patients in the EPC trial, there is an
irrefutable positive result. Bicalutamide 150 mg decreases the number
of bone scan events compared to placebo. When the patients are stratified
by risk, the benefits from two years of adjuvant bicalutamide are
less in patients with low-risk disease and greater in patients with
high-risk disease. I certainly have a greater comfort level offering
bicalutamide 150 mg to high-risk individuals whom I believe are
going to benefit even in the short term, rather than to low-risk
individuals who have a greater likelihood of being cured by the
primary therapy. However, that may change with time.
Urologists have been “behind the eight ball” with
regard to considering multiple treatment options and multiple prognostic
factors. In the last decade, urologists have begun to think about
different treatment options for localized disease. We are getting
better at teaching patients about surgery, radiation and brachytherapy.
Last year at the AUA meeting, there was a lot of buzz with regard
to risk assessment.
Since the Partin tables were developed for staging, more urologists
are thinking about multiple factors to predict risk. This will translate
into thinking about risk assessment and realizing that monotherapy
will not cure these high-risk patients. Over the next five years,
there will be a further understanding of risk assessment and adjuvant
therapy options.
Until recently, urologists believed that many patients had localized
disease. Now there is recognition that a lot more patients have
micrometastatic disease than we originally thought. The only adjuvant
therapy that urologists thought about was traditional hormonal therapy.
As surgeons, we pride ourselves in diagnosing these men early and
performing nerve-sparing prostatectomies to maintain sexual function.
We do not want to compromise sexual function. Since we know traditional
hormonal therapy will impact on their sexual function, we are reluctant
to recommend it. We need to start thinking about other hormonal
therapy options that may not have as much of an effect on sexual
function.
Select publications
|