 Home: PCU 5|2003: Michael K Brawer, MD Home: PCU 5|2003: Michael K Brawer, MD
 |
|
Michael K Brawer, MD |
| |
| |
Director, Northwest Prostate Institute,
Northwest Hospital |
Edited comments by Dr Brawer
Intermittent androgen suppression in a man with postprostatectomy biochemical recurrence
History
At the end of 1998, this healthy 59-year-old man had a screening total PSA of 17.3 ng/mL and a complexed PSA of 14.7 ng/mL. On examination, he had a 30 cc benign gland. We performed an ultrasound-guided prostate biopsy with eight cores, including two transition zone cores. He had cancer in all but one of the cores; the majority of the cancer was Gleason 3+3, and one core from the left base was Gleason 3+4. His bone scan was negative and there was moderate heterogeneous activity in the prostate and a mildly suspicious right iliac lymph node on the ProstaScint® scan.
After a discussion of treatment options, he elected to undergo radical prostatectomy. Based on the number of positive cores with a high grade, I did not do a nerve-sparing procedure. Because of the results of the ProstaScint® scan, I did an extensive lymphadenectomy, which was negative. His final pathology revealed a 9.9 cc, Gleason 3+4 prostate cancer with bilateral seminal vesicle invasion.
Follow-up
We discussed the options of adjuvant radiation or hormonal therapy or an experimental chemotherapy protocol. He elected to watch his PSA prior to making any decisions about adjuvant treatment. Four months after the prostatectomy, his postoperative PSA reached its nadir at 0.2 ng/mL, and then it began to very slowly rise to 0.5 ng/mL 10 months after the prostatectomy. At that point, we again discussed possible interventions, and we started intermittent hormonal therapy with the LHRH agonist. Over the 42 months that he has been followed since his initial rise in PSA after surgery, he has received 18 months of active therapy and has been off of therapy more than half of the time.
Despite the non-nerve-sparing procedure, he maintains his potency. Even more amazing and unique in my personal series, he maintains his potency on the LHRH agonist. He must have an accessory location of some of the nerves. When he's on the LHRH agonist, he has some emotional issues, such as feeling less competitive in sports. Otherwise, he's done quite well.
Discussion
When we initially discussed hormonal therapy options, we also talked about continuous hormonal therapy. I presented the data from the Vancouver Group and went over the potential option of the intermittent approach. My current interpretation of the data suggests there is no detrimental effect, some improvement in quality of life and an economic benefit associated with intermittent androgen suppression.
We were interested in his amazing libido and potency through all of the treatments, and we checked his testosterone levels. When he's on the LHRH agonist, he becomes castrate almost immediately, and he has a rapid restoration of his testosterone level following the LHRH agonist. If he receives a three-month LHRH injection, by five months his testosterone is back to his normal level. Particularly in older patients, I see that most of the LHRH agonists have a more prolonged duration of castration than the prescribed three- or four-month interval.
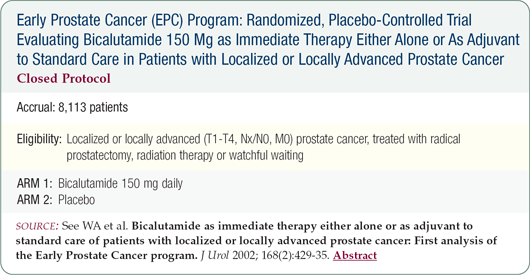
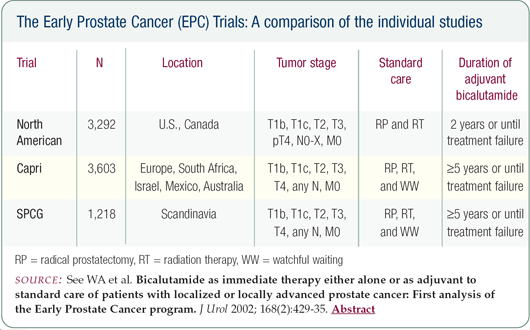
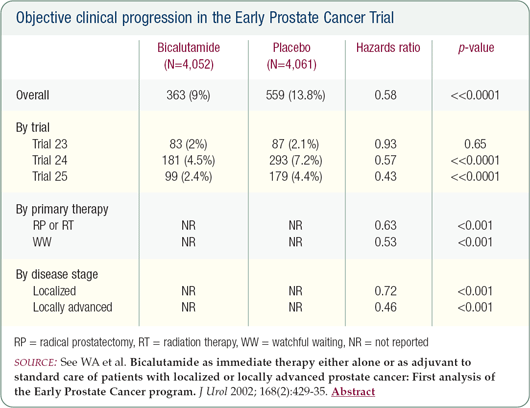
After the impressive results from the Early Prostate Cancer bicalutamide trial, if this patient presented today, he would be an excellent candidate for high-dose bicalutamide. I've discussed switching him to bicalutamide, but he declined because he says he has done so well. If the LHRH agonist eventually affects his libido, switching to bicalutamide may be a reasonable option.
Given the data from the bicalutamide trial, I would now recommend this agent as first-line therapy immediately after having found the aggressive nature of his cancer on the pathology specimen.
Local therapy in high-risk situations
This man presented in 1998. Today, in patients such as this, I've abandoned radical prostatectomy as first-line therapy and recommend neoadjuvant and adjuvant hormonal therapy in conjunction with external beam radiation and brachytherapy. The patients are treated with three months of an LHRH agonist, followed by brachytherapy and then by external beam radiation. Thirty percent of the patients will receive an antiandrogen, such as bicalutamide, one week before the LHRH agonist. Patients will continue on the hormonal therapy for a year. That is a departure from some of the studies, but it's the way we've approached these patients.
PSA as a surrogate endpoint
With the development and refinement of PSA, we have an extraordinarily powerful tool for the detection and monitoring of prostate cancer. Hopefully, the regulatory agencies will begin to look at PSA and other intermediate endpoints as useful data for the approval of therapies.
PSA nadir and the length of time to the PSA nadir are not sufficient endpoints for the FDA or other agencies to support the approval of a novel therapy. However, the preponderance of evidence suggests that PSA is an extraordinarily useful tumor marker to predict an individual patient's outcome, as long as the therapy does not affect PSA independently of the tumor. This is, of course, a potential problem for PSA and hormonal therapy, because the androgen receptor regulates PSA gene transcription.
With respect to hormonal therapy, PSA outcome may be somewhat problematic. As we move to other therapies that don't affect the upstream transcription of PSA, we ought to use PSA and potentially other markers as useful surrogate endpoints. It would dramatically decrease the time and expense of clinical trials and allow us to develop novel therapies more rapidly.
Complexed PSA
While PSA has relatively good sensitivity, its specificity is problematic. The majority of men with an elevated PSA will not have prostate cancer, at least not on their initial biopsy. On that basis, we have spent a great deal of time trying to make PSA more specific. We've gone through a whole host of machinations, starting with the PSA derivatives (PSA velocity, age-specific PSA and PSA density) and then moving on to the molecular forms of PSA (free PSA and complexed PSA). A number of papers presented at this year's American Urological Association meeting support the benefit of complexed PSA relative to total PSA, both for initial testing and monitoring of patients.
PSA in ejaculate is completely in the free form, and the vast majority of PSA in the systemic circulation is complexed with protease inhibitors, including alfa-2-macroglobulin and, most importantly, alfa-1-antichymotrypsin. It is not known why this occurs, but we do know that the free form occurs in a greater percentage of men without prostate cancer and the complexed form occurs in a greater number of men with prostate cancer.
For a number of years, the free-to-total PSA ratio was calculated. This provided some enhancement in the test's specificity, but it had a number of problems. First, there was disagreement between the different manufacturers in what their assays read for a given patient's specimen. Also, the free form of PSA was not very stable and required meticulous specimen handling. Most importantly, the free-to-total PSA ratio required the measurement of both the free and the total PSA. With the complexed PSA, we obviate the problem of stability, and we only need to measure the complexed PSA. Therefore, we don't need to measure two analytes, and the cost of PSA testing is cut in half.
Reduction in prostate cancer mortality
PSA has now been widely used for 17 or 18 years, and it may be that some of the reduction in prostate cancer mortality is related to early detection and definitive therapy for local disease. A question that will be answered through the Prostate, Lung, Colorectal and Ovary (PLCO) cancer trial is: Does conventional local therapy truly change the course of disease? In the Scandinavian trial, while prostate cancer mortality was reduced in men undergoing radical prostatectomy, the all-cause mortality was the same for men treated with "watchful waiting" or definitive therapy.
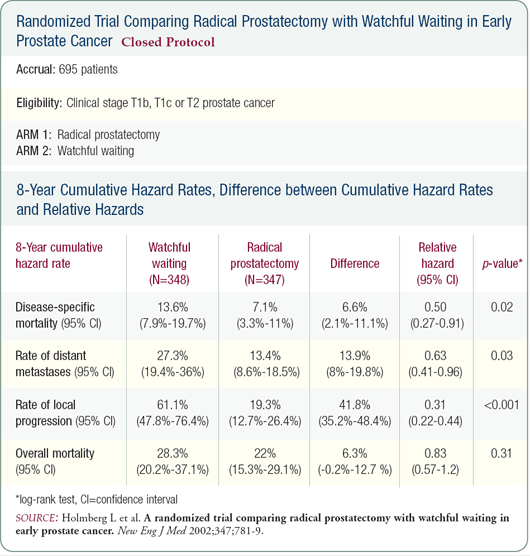
The Prostate Cancer Intervention Versus Observation Trial (PIVOT) is a U.S.-based trial that randomizes men to radical prostatectomy or observation. Unlike the Scandinavian trial, PIVOT reflects the contemporary U.S. experience where PSA is the basis for the diagnosis in the majority of patients. Unfortunately, we will not know the results for many years. With PIVOT and the PLCO trial, we'll know whether early detection contributes to the reduction in prostate cancer mortality.

Select publications
|