 Home: PCU 5|2003: Michael J Zelefsky, MD Home: PCU 5|2003: Michael J Zelefsky, MD
 |
|
Michael J Zelefsky, MD |
| |
| |
Chief, Brachytherapy Service,
Memorial Sloan-Kettering Cancer Center |
Edited Comments by Dr Zelefsky
Trends in the use of radiation therapy for prostate cancer
Tremendous evolution has occurred in the use of radiation therapy in the treatment of prostate cancer, specifically with respect to the different types of radiation and adjuvant therapies. In the Patterns of Care Survey conducted by the American College of Radiology, we observed a dramatic shift over the last five to 10 years.
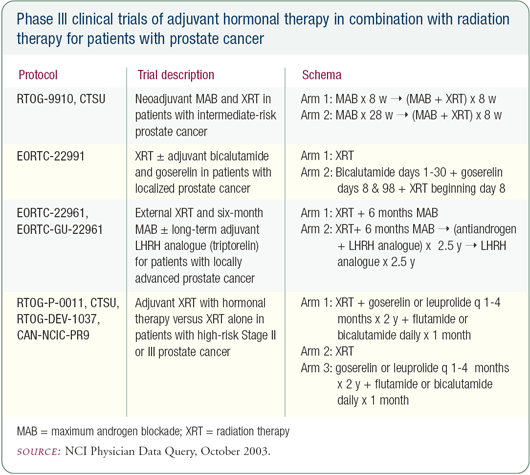
One trend is the increase in frequency of hormonal therapy in combination with radiation therapy. This is based on a number of randomized trials demonstrating a benefit for that combination, and these trials have also evaluated different ways of sequencing hormonal therapy (i.e., prior and concurrent, or after completion of radiation therapy). These trials demonstrated that hormonal therapy with standard radiation therapy seems to provide better outcomes (i.e., reduced PSA progression and reduced likelihood of distant metastases).
We also have new, more sophisticated, precise and targeted radiation therapy modalities; therefore, we're seeing fewer side effects. The emergence of 3-dimensional (3D) conformal radiotherapy and intensity-modulated radiation therapy (IMRT) has had a major impact on the treatment of prostate cancer. We can deliver unprecedented doses of radiation, and many studies have shown these higher doses translate into better results in terms of PSA control and other outcome parameters.
These new radiation therapy techniques are more exact, and we're seeing less morbidity and toxicity. This clearly has had an impact on the quality of life of these patients. In the last 10 years, interest and enthusiasm for brachytherapy has emerged, and we are seeing fewer side effects and better control rates with this technology.
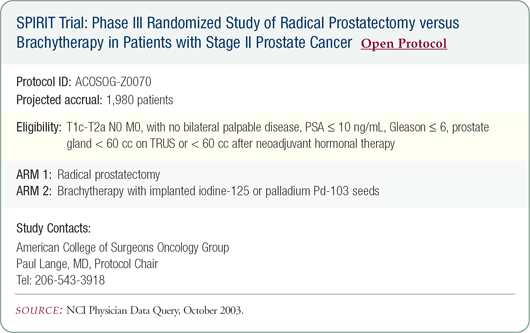
SPIRIT trial: Brachytherapy versus radical prostatectomy in early-stage disease
We have no randomized trial data to help patients in the selection of optimal local therapy. The only way to know whether one therapy is better is to conduct a randomized trial comparing various treatments for patients with localized prostate cancer, although many believe it will be impossible to conduct such a trial in the United States.
I give a great deal of credit to the principal investigators of the SPIRIT trial. Great efforts have been made to optimize quality assurance within the trial so that optimal seed implant techniques and prostatectomy surgical techniques are used.
I support the trial, and we participate at our institution. While it is an important study, and I hope it accrues well, it will be difficult to accrue patients in certain parts of the country. For instance, it's not easy to enroll my patient population in such a trial. Many patients have preconceived notions about how they would like their prostate cancer treated, and they are reluctant to be randomized.
I've spoken with some of the principal investigators of the SPIRIT trial and have learned that accrual rates improve in settings in which patients are able to speak with a multidisciplinary team about the pros and cons of each of the therapies. When patients see that their own physicians have uncertainties about optimal therapy, they are more likely to consider participation.
Brachytherapy in combination with external beam radiation
The combination of external beam radiation therapy and brachytherapy is an excellent way to deliver a high dose of radiation to the prostate. Numerous studies demonstrate that higher doses of radiation translate into better results.
Not all patients, however, need this form of therapy. A patient with early-stage disease, a PSA less than 10 ng/mL, a Gleason score less than or equal to 6 and low volume disease could be effectively treated with external beam radiation therapy alone or brachytherapy alone. In patients with intermediate-risk disease and some aggressive features, or in patients with unfavorable-risk disease, higher doses are necessary.
Combining radiation therapy with hormonal therapy
At our institution, we have launched a Phase III trial randomizing patients with higher risk features to high-dose radiation therapy alone or lower doses of radiation therapy plus hormonal therapy. The question is: Will a high enough dose of radiation obviate the need for hormonal therapy in patients with more aggressive features?
All of the randomized trials demonstrating benefits from hormonal therapy in conjunction with external beam radiation therapy have used suboptimal radiation therapy doses. The questions are: In the setting of hormonal therapy, do we need these higher doses of radiation? If we use these higher doses of radiation, is hormonal therapy still necessary?
Among patients who have undergone biopsy years after treatment with radiation and hormonal therapy, we have seen an improved likelihood of disease eradication from the prostate. Hormonal therapy may have a radiosensitizing effect. In addition, randomized trials have shown a decrease in distant metastases among patients treated with hormonal therapy in combination with radiation. It may be argued that this simply represents a delay in the manifestation of distant metastases, or it could mean that some of these small tumor clones were prevented from disseminating. I believe that, especially in patients with high-risk disease, hormonal therapy may have both a local and a systemic effect.
Selection and timing of hormonal therapy
When prescribing hormonal therapy, we usually use an LHRH agonist and one month of an antiandrogen. Generally, we use the hormonal therapy prior to and in conjunction with the radiation therapy. For those patients with a Gleason score greater than or equal to 8 or those with T3 disease, we recommend at least six to 12 months, and more often two years, of adjuvant hormonal therapy based on the RTOG randomized trials.
PSA "bounce"
After radiation therapy, there is a "bounce" phenomenon. Over a period of two to three years, patients will have some natural fluctuations in their PSA level, which cause a great deal of anxiety. Patients need to be reassured about how their PSA will behave in the postradiation therapy period. If patients are prepared for the PSA fluctuations, they will certainly have less anxiety. Without hormonal therapy, it takes about 12 to 18 months for the PSA to gradually drop to its nadir. In some patients, the PSA continues to decrease for years after the completion of treatment. There may be occasional "bounces" here or there, which sometimes respond to antibiotic therapy or may gradually go down with time.
In a patient with a rise in PSA during the postradiation period, we recommend obtaining another PSA in about three to four months and follow it over a period of time to establish the PSA kinetics. If there are minor fluctuations in the PSA, we try to reassure the patient and do not initiate any particular therapy.
Personal research interests in radiation oncology
I have an interest in the role of intensity-modulated radiation therapy. We have been working for many years on dose escalations with these new conformal technologies and have demonstrated improved outcomes in terms of PSA relapse-free survival with these higher doses. These new technologies are now being used to reduce potential toxicities.
We are also looking at dose painting. This technique allows us to use imaging technologies such as MR spectroscopy and PET imaging to identify within the gland where tumor clones may be most abundant. We can then focus or intensify the radiation doses to those particular zones in the prostate. This may have a significant impact on further reducing toxicity. Dose painting allows us to target particular areas of the prostate with more intense doses while sparing the urethra and the rectum.
I also have a research interest in the utilization of new technologies in brachytherapy that further enhance the targeting of the seeds and reduce the toxicity. We've been working on intraoperative, computer-based technologies that provide feedback as to exactly where these seeds should be placed. This technique optimizes the placement of the seeds, helps reduce the dose to the urethra and ensures optimal coverage of the prescription dose to the prostate, resulting in a better quality implant.
We're also currently working on sophisticated modalities that give the operator feedback as to where the seeds are placed, so we can continuously modify the implant plan. Theoretically, before walking out of the operating room, you have the perfect implant. This is known as dynamic dosimetry. For the first time, the operator really has a handle on where the seeds are being dropped to ensure a very accurate implant.
Select publications
|