|
 Home: PCU 4|2004: Laurence Klotz, MD Home: PCU 4|2004: Laurence Klotz, MD

 |
Antiandrogens are Androgen Receptor Antagonists:
Clinical Implications for the Use of Maximal Androgen Blockade |
 |
Laurence Klotz, MD |
| 3.1 |
|
| |
|
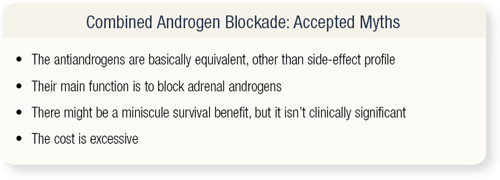
SLIDE 3.1 The accepted myths about combined androgen blockade (CAB) are basic. “The antiandrogens are equivalent, other than their side-effect profiles.” Their main function is to block adrenal androgens, so the term “antiandrogen” is a misnomer and I’ll come back to that. There might be a miniscule survival benefit, but it isn’t clinically significant, and the cost is excessive. Hopefully, I’ll dissuade you about each one of these myths.
|
| |
|
|
| 3.2 |
|
| |
|
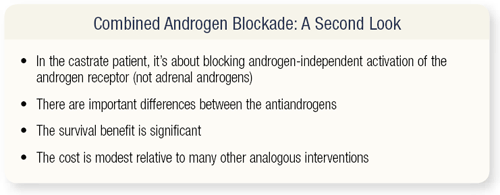
SLIDE 3.2 Why is CAB worth a second look? Four reasons exist. In the castrate patient, whom I’m talking about, the adrenal androgens are almost certainly not the major issue. It’s androgen-independent activation of the androgen receptor that’s antagonized by the nonsteroidal antiandrogens that’s important. A lot of work has been done in these last few years that’s not well known, and I’ll review it very briefly.
The nonsteroidal antiandrogens have quite different actions in terms of the degree to which they block androgen-independent activation. The survival benefit is significant, and there’s a new analysis I’m going to show with bicalutamide, which demonstrates that it’s probably much more significant than has been recognized. The cost is modest relative to other analogous interventions.
|
| |
|
|
| 3.3 |
|
| |
|
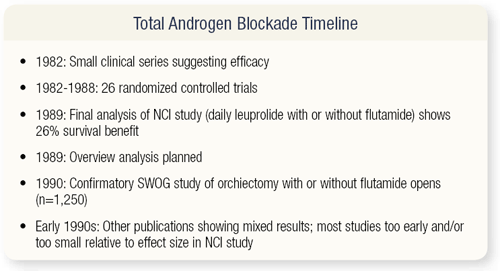
SLIDE 3.3 The timeline for CAB is well known, going back to 1979 and the first publications by Labrie. This is the single most-studied question in urology, as far as I know, with 26 randomized controlled trials. The NCI study, with a 26 percent survival benefit, changed clinical practice. Everyone started using CAB, and in the late 1980s the Overview analysis under Richard Peto’s direction was planned. The SWOG study was powered based on the expectation of finding a 25 percent survival benefit. In the 1990s, clinical trials had mixed results.
|
| |
|
|
| 3.4 |
|
| |
|
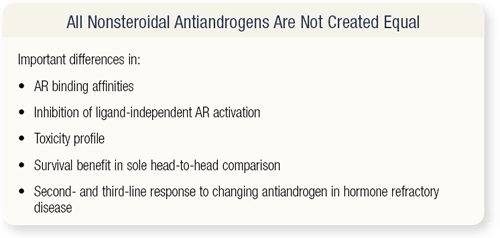
SLIDE 3.4 So, where are we today? All nonsteroidal antiandrogens are not created equal. Differences exist in binding affinities, inhibition of ligand-independent AR activation, the toxicity profiles, survival benefits in the sole head-to-head comparison, and second- and thirdline responses to changing antiandrogen therapy in patients with hormone refractory disease.
|
| |
|
|
| 3.5 |
|
| |
|
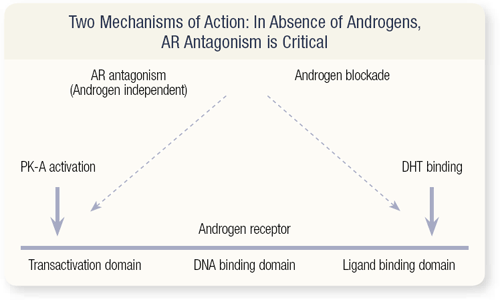
SLIDE 3.5 If these drugs were the equivalent of the alpha blockers — alfuzosin versus doxazosin versus tamsulosin — you wouldn’t expect to see the second-line responses, where patients taken off one antiandrogen responded to another. A lot of evidence exists that they do have different actions. So, the term “antiandrogen” is a misnomer. These agents really are androgen receptor antagonists, meaning they not only competitively block ligand binding at the ligand binding domain, but they also block androgen-independent activation.
This is mediated by protein kinase A, and there’s a whole slew of cytokines — IL12, IL8, TGF beta — that can activate the androgen receptor in the absence of the ligand. And this is blocked by the antiandrogens. They also interact with co-activators and co-suppressors of the androgen receptor, and the ideal antagonist would inhibit co-activators and activate co-repressors. So, these are just two pieces of laboratory data demonstrating differences in the androgen-independent activation blockade.
|
| |
|
|
| 3.6 |
|
| |
|

SLIDE 3.6 Finally, the single head-to-head comparison of flutamide and bicalutamide was first published about six years ago with 800 patients who had D2 disease, and the median follow-up was approximately three years. The population in this study was very comparable to the patients who were entered on the earlier MAB versus monotherapy studies — D2 and advanced disease. The study demonstrated leuprolide plus flutamide was inferior to the other three groups, and bicalutamide was superior to flutamide.
In this two-by-two study, if you pull out the bicalutamide and flutamide arms plus LHRH, there is a significant overall survival benefit for the bicalutamide compared to the flutamide. That’s only one study, but it was a large and well-conducted study.
|
| |
|
|
| 3.8 |
|
| |
|
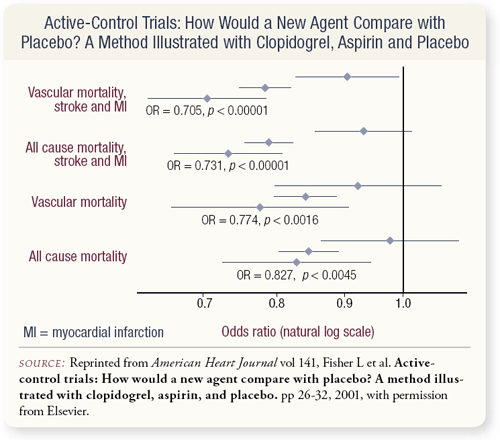
SLIDES 3.7, 3.8 So, the question is: Can you somehow integrate this data with all the MAB data to draw conclusions about the benefit of bicalutamide compared to monotherapy? Well, you wouldn’t think so. The conventional wisdom is you can’t compare data from different trials, but it turns out it can be done. Biostatisticians have attempted to define models to allow these comparisons, and several reports in the literature exist where drugs actually received FDA approval based on the demonstration of benefit from these models. This type of modeling demonstrated that drug X is greater than drug Y and drug Y is greater than drug Z from earlier clinical trials, therefore it can be concluded that drug X is superior to drug Z.
The problem we face in this setting, where no hormone-naïve D-2 patients are available to study anymore, is analogous to other situations in medicine where you have comparisons that were done five or ten years ago that are no longer possible. In one example, capecitabine compared to 5-FU plus leucovorin showed a survival benefit, and the question was: Is capecitabine superior to 5-FU alone? Studies demonstrated 5-FU plus leucovorin was superior to 5-FU alone. So, this was submitted to the FDA, and capecitabine was approved as being superior to 5-FU alone, even though it had never been directly compared.
Another example involves a comparison of the platelet antagonist clopidogrel to placebo. Clopidogrel was shown to be superior to aspirin. Aspirin is superior to placebo in terms of recurrence, cardiovascular mortality and survival benefit. To make a long story short, it can be seen that clopidogrel was superior to placebo, even though they’ve never been directly compared, and yet this kind of analysis was accepted. The basic posit is that the patients have to be comparable, and the mathematics are complex in terms of figuring out the confidence limits.
|
| |
|
|
| 3.9 |
|
| |
|
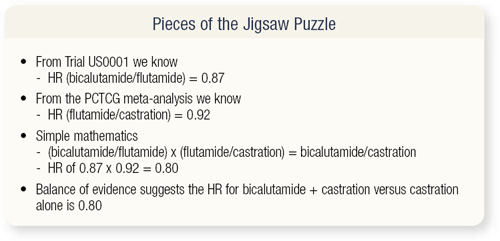
SLIDE 3.9 In the Schellhammer trial, comparing bicalutamide to flutamide, the hazard ratio was 0.87, and in the PCTCG meta-analysis, comparing flutamide to castration, the hazard ratio was 0.92. The mathematics of a hazard ratio of 0.87 multiplied by 0.92 is 0.80, which is based on randomized trials. The D2 patients in the Schellhammer trial are comparable to the D2 patients in the MAB analysis, and to my mind, there’s no reason they would respond differently to MAB than the patients in the earlier trial. So, the balance of evidence suggests the hazard ratio for bicalutamide plus castration versus castration alone is 0.8. That’s a significant survival benefit by anyone’s standard.
|
| |
|
|
| 3.10 |
|
| |
|
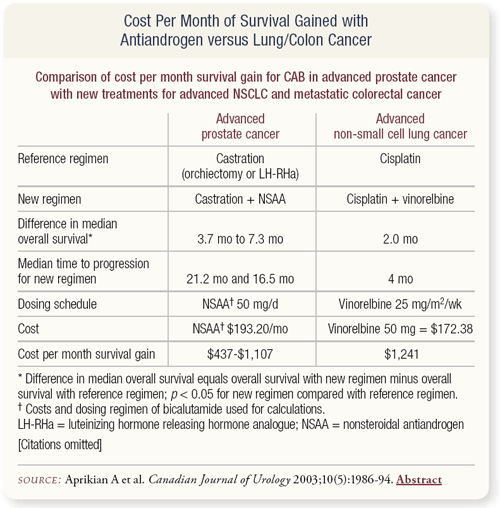
SLIDE 3.10 What about the cost of therapy? A report by Armen Aprikian and colleagues in the October 2003 issue of the Canadian Journal of Urology compared the cost of MAB with flutamide, using the survival benefit from the PCTCG meta-analysis of three to seven months compared to other interventions for advanced small-cell lung cancer, metastatic colon and breast cancer. The interventions included vinorelbine, irinotecan, trastuzumab, and anastrozole.
Basically, in terms of cost per month of survival gain — even if you accept it’s three to six months, much less six to 12 months, which one would expect for D2 prostate cancer — the conservative assessment would be $500 per month versus up to $11,000 with irinotecan and $5,000 with trastuzumab. So, the cost compared to other interventions that are used by medical oncologists is relatively modest.
|
| |
|
|
| 3.11 |
|
| |
|
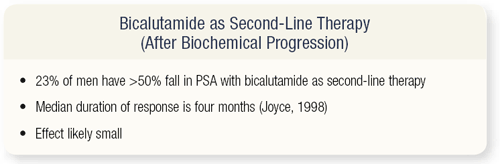
SLIDE 3.11 So, the question that arises is why not just use MAB as second-line therapy? Why not just treat when patients progress and get the same effect? The point is that the second-line responses tend to be short, and roughly only one in four patients will derive more than a 50 percent PSA response with bicalutamide with a median response duration of four months. So, you’re unlikely to see a very significant overall survival benefit with these agents used as second-line therapy.
|
| |
|
|
| 3.12 |
|
| |
|
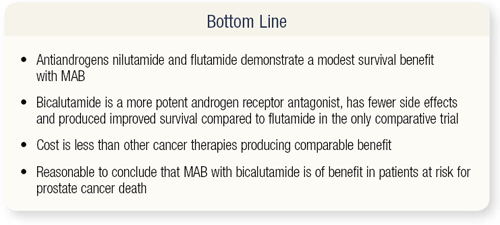
SLIDE 3.12 The bottom line is a modest survival benefit conferred by the older drugs. Bicalutamide is a more potent androgen receptor antagonist, with fewer side effects and improved survival in the only comparative trial. The cost is less than other cancer therapies and results in comparable benefits. It’s reasonable to conclude that MAB with bicalutamide will benefit patients at risk for prostate cancer death, and patients who aren’t at risk of death shouldn’t be treated.
|
| |
|
|
Select publications
|
|
|
|
|
|
|
|
|
|