|
 Home: PCU 4|2004: Judd W Moul, MD, FACS Home: PCU 4|2004: Judd W Moul, MD, FACS

 |
Management of PSA Relapse:
Early versus Late Androgen Deprivation |
 |
Judd W Moul, MD, FACS |
| 2.1 |
|
| |
|
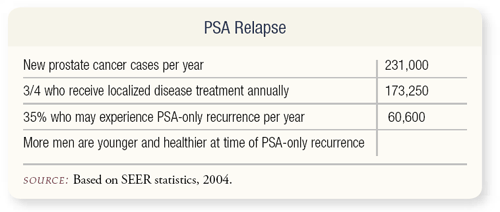
SLIDE 2.1 Depending on how you define it, rising PSA may be the most common stage of advanced prostate cancer. Certainly, the quality of life slips when PSA rises after prostatectomy, brachytherapy or external beam radiation.
|
| |
|
|
| 2.2 |
|
| |
|
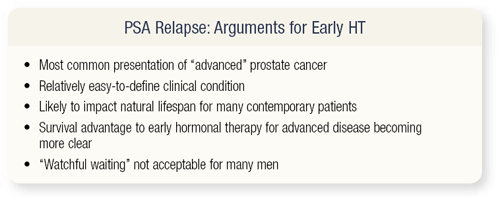
SLIDE 2.2 Several arguments exist for early hormonal therapy: PSA relapse is the most common presentation in advanced disease, it’s a relatively easy-to-define clinical condition, and it’s likely to impact the natural life span of many contemporary patients. Early hormonal therapy for advanced disease offers a survival advantage that is becoming more clear, and watchful waiting is not acceptable to many of our patients.
|
| |
|
|
| 2.3 |
|
| |
|

SLIDE 2.3 Arguments against early hormonal therapy include the long natural history of a rising PSA before clinical metastases and death for most men. Additionally, no randomized clinical trials have been conducted to address this issue, which is a major problem. Finally, the side effects and cost of hormonal therapy are not inconsequential.
|
| |
|
|
| 2.4 |
|
| |
|
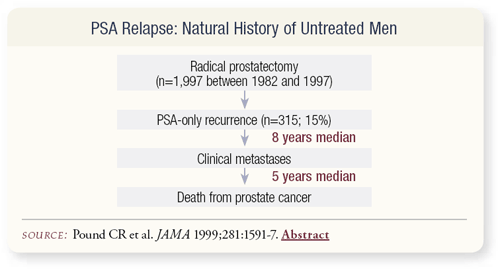
SLIDE 2.4 The Pound article from Johns Hopkins describing the wellknown eight-year median time from PSA recurrence to clinical metastases is based on the Hopkins’ definition of 0.2 ng/mL. At the time of clinical metastases, most of those men began hormonal therapy and had a median survival of five years from initiation of therapy until death, so the average survival after PSA recurrence was 13 years.
|
| |
|
|
| 2.5 |
|
| |
|
SLIDE 2.5 A study we conducted through the Center for Prostate Disease Research (CPDR) was published in the March 2004 issue of the Journal of Urology. We examined the CPDR database in an attempt to determine what was happening to patients with PSA-only recurrences in the military healthcare system.
|
| |
|
|
| |
|
|
| 2.6 |
|
| |
|
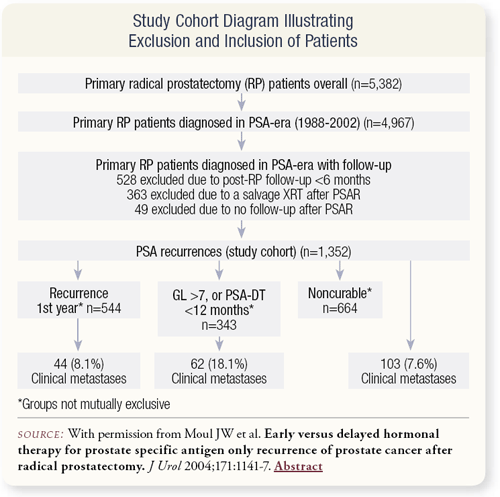
SLIDE 2.6 The database included 5,382 patients who underwent primary radical prostatectomy, of which 4,967 occurred during the PSA era — 1988 to 2002. We excluded 528 patients due to inadequate postradical prostatectomy follow-up (less than six months) and 363 patients due to salvage radiation therapy after a PSA recurrence. We could argue whether that was valid or not, but we were trying to duplicate the Pound study, which excluded patients who underwent salvage radiation therapy. An additional 49 patients were excluded due to lack of follow-up after the PSA recurrence.
Defined by the Pound criteria of a PSA greater than 0.2 ng/mL, 1,352 patients had PSA recurrences. We also performed some subset analyses, evaluating the subgroup of 544 patients who had a recurrence in the first year, the subgroup of 343 patients at high risk who had a Gleason greater than seven in their radical specimen, or a PSA doubling time less than 12 months and a third subgroup analysis of noncurable patients defined by the Hopkins’ definition of noncurable.
Overall, of these 1,352 patients, 103 had clinical metastases, which was the endpoint for the study. In the high-risk group of 343 patients, 62 had clinical metastases. Of the patients with recurrence in the first year, 44 had clinical metastases. The average follow-up was about four years after PSA recurrence.
|
| |
|
|
| 2.8 |
|
| |
|
SLIDE 2.7 Breaking down these 1,352 PSA recurrences, 221 patients started hormone therapy for a PSA greater than 0.2 ng/mL but less than 2.5 ng/mL; 47 patients for a PSA between 2.6 ng/mL and 5 ng/mL; 39 patients for a PSA between 5 ng/mL and 10 ng/mL; and then 48 patients for a PSA greater than 10 ng/mL but before clinical metastases. The remaining 997 patients, with a median follow-up of almost five years after radical prostatectomy, received no hormonal therapy.
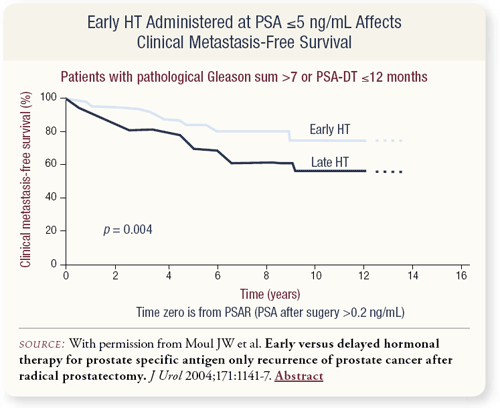
SLIDE 2.8 Early hormonal therapy conferred a clinical metastasis-free survival benefit in the group of patients with a pathologic Gleason’s sum greater than seven or a PSA doubling time 12 months or less in men who started hormonal therapy before their PSA reached 5 ng/mL.
|
| |
|
|
| 2.10 |
|
| |
|
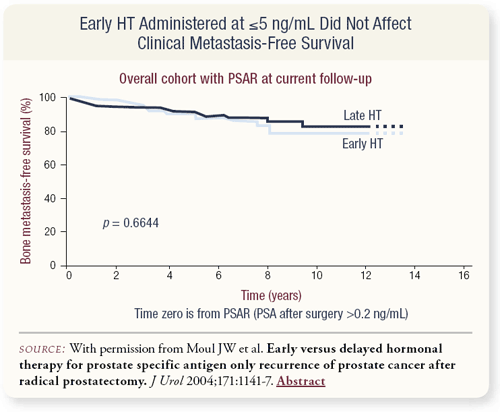
SLIDES 2.9, 2.10 Patients who had a PSA less than 10 ng/mL, as opposed to less than 5 ng/mL, had similar benefit from early hormonal therapy, although whether you started at less than 5 ng/mL or less than 10 ng/mL, in this type of database analysis we couldn’t demonstrate a benefit for patients at high risk. In the overall cohort of over 1,300 patients, however, there was no clinical metastasis-free survival benefit from early versus delayed hormonal therapy.
|
| |
|
|
| 2.12 |
|
| |
|
SLIDE 2.11 The good news is this is the first study to show a clinical disease-free survival benefit from early hormonal therapy for PSA recurrence. I’m almost embarrassed to say that we should have a randomized trial in this group of patients, and this retrospective database study is really the first such paper in the literature. The findings emphasize the importance of risk stratification in patients with PSA relapse and tend to support that men with high-grade disease and a short PSA doubling time are at high risk for clinical failure.
The bad news is this was not a randomized, controlled trial. Overall, if we look at all 1,300 patients with PSA recurrence, early hormonal therapy showed no benefit. This is a database study and is a moving target.
The results may change over time as the patients are followed and more of those nonhormonal therapy patients switch to hormonal therapy. Currently, the follow-up is too short to determine if an overall survival impact exists.
In the last couple of slides, I want to emphasize the importance of risk stratification in patients with biochemical recurrence.
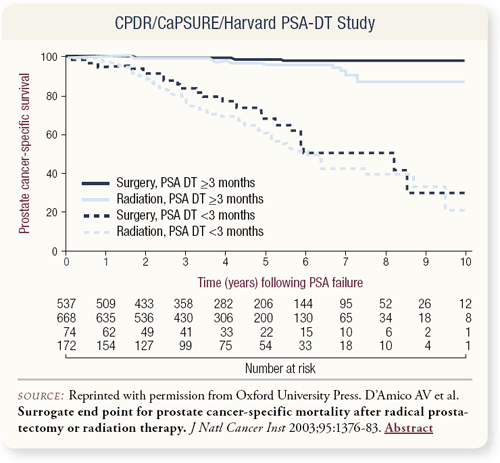
SLIDE 2.12 Anthony D’Amico, Peter Carroll and I combined our databases to evaluate PSA doubling time and demonstrate that a PSA doubling time of less than three months was a surrogate for death from prostate cancer. Among the factors we examined, PSA doubling time definitely seems to be an endpoint we should be using as we design future clinical trials.
|
| |
|
|
| 2.13 |
|
| |
|

SLIDE 2.13 The take-home message is: PSA relapse is very common and no randomized controlled trial data exist to guide our clinical decisions. Our recent work emphasizes that we must take a risk-stratified approach to PSA relapse. Men with high-grade disease and those with a short PSA doubling time appear to have delayed clinical metastases if they receive early hormonal therapy. It’s unknown whether early hormonal therapy for PSA relapse will improve cancer-specific or overall survival. That will require longer follow-up in the CPDR database.
|
| |
|
|
Select publications
|
|
|
|
|
|
|
|
|
|