|
 Home: PCU 4|2004: Ian M Thompson, MD Home: PCU 4|2004: Ian M Thompson, MD

 |
The Prostate Cancer Prevention Trial Revisited |
 |
Ian M Thompson, MD |
| 4.1 |
|
| |
|

SLIDE 4.1 Why prevent prostate cancer? Prevention certainly has a number of advantages, one of which is that it obviates the issue of overdetection and overdiagnosis, and has an enormous impact on health. Every one percent reduction in prostate cancer prevalence reduces treatment for prostate cancer by 2,000 patients a year in the United States.
Another favorable aspect of prevention is that in many cases the carcinogenesis appears to occur over a period of decades. Most prostate cancer-related deaths occur late in life, so you don’t necessarily have to prevent the disease — if you can delay it, perhaps by five to seven years, you may actually cut mortality in half.
|
| |
|
|
| 4.2 |
|
| |
|
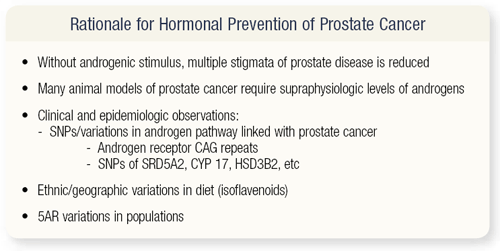
SLIDE 4.2 Why hormonal prevention? We know that androgenic stimuli are associated with prostate disease and carcinogenesis. Preclinical models frequently require supraphysiologic levels of androgens. The EPI data suggest that anything that makes the androgen receptor see more stimulation leads to a higher risk of prostate cancer. A large body of evidence indicates that variations among ethnic groups by diet or by behavior is related to risk and binding with the androgen receptor.
Certainly, before the development of 5-alpha-reductase inhibitors, we really didn’t have a good preventative approach — other than perhaps switching to an Asian diet — to reduce one’s risk of disease. The development of 5-alpha-reductase inhibitors provided us with new opportunities.
|
| |
|
|
| 4.3 |
|
| |
|
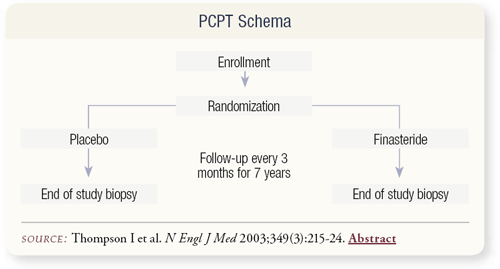
SLIDE 4.3 The PCP trial was designed in 1992 and began enrollment shortly thereafter. The target accrual was 18,000 men over a period of three years, who would then be followed annually with rectal examinations and PSA tests. Due to the impact of finasteride on PSA, a biopsy was to be performed at the end of the study, which is probably the most interesting aspect of the trial.
|
| |
|
|
| 4.4 |
|
| |
|
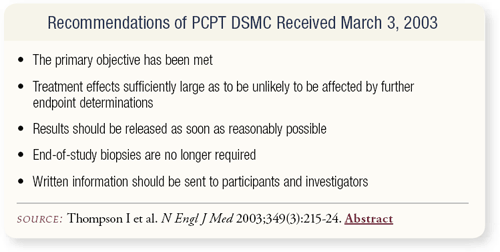
SLIDE 4.4 The Data and Safety Monitoring Committee of PCPT met in early 2003, and the results of their deliberations were released in March. The trial was closed approximately 15 months early because the primary objective, which was a reduction in the prevalence of prostate cancer, had been met. Additional biopsies were unnecessary. Participants and investigators were notified, and the results were published in the July 2003 issue of the New England Journal of Medicine.
|
| |
|
|
| 4.5 |
|
| |
|
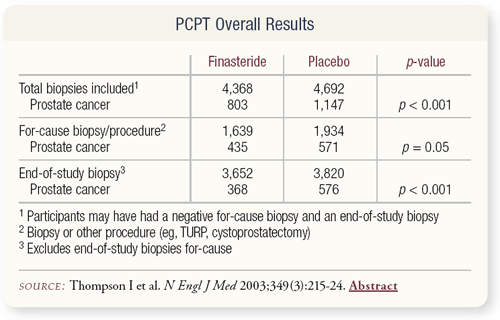
SLIDE 4.5 A 25 percent reduction in prostate cancer prevalence was observed over the course of the trial. We wanted to analyze two sets of biopsies — the for-cause prostate diagnoses, which were prompted by an abnormal rectal examination or a PSA above 4 ng/mL, and the end-of- study biopsies, which is this kind of anomalous category of prostate cancer that we didn’t know existed.
|
| |
|
|
| 4.6 |
|
| |
|
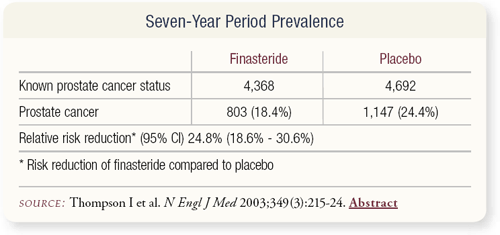
SLIDE 4.6 A significant reduction in prostate cancer occurred in both groups, and we diagnosed prostate cancer in a fair number of patients to whom we would routinely say, “You don’t have prostate cancer.”
|
| |
|
|
| 4.9 |
|
| |
|
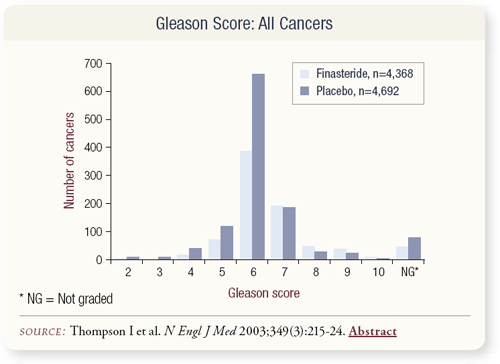
SLIDES 4.7 – 4.9 The biopsy technique required a minimum of six cores. The trial was designed in 1992, and we anticipated a bias would exist as a result of that, because of the volume reduction in the finasteride arm and potential overdetection in the finasteride arm.
In fact, four or five years ago we asked ourselves, “Should we require more biopsies in the placebo arm to equalize detection?” We decided that would introduce another bias, and we assumed that any bias would be against the finasteride arm and any beneficial effect observed would be potentially greater.
One of the most interesting anomalies in this trial was a substantial reduction in Gleason 5 and 6 prostate cancer, which were the predominant numbers, but effectively not much change in Gleason 7, and 47 more cases of Gleason 8-10 cancer in patients who received finasteride.
Approximately 50 percent of all prostate cancer diagnosed in the United States today is Gleason 6. If you examine the individual Gleason scores, you see substantial reductions in Gleason 6 cancer — more than 300 fewer cases — and a slight increase in Gleason 8, 9 and 10 cancers.
|
| |
|
|
| 4.11 |
|
| |
|

SLIDES 4.10, 4.11 The real question is whether the differential effect on grade is real or an artifact? If it’s real, then it’s a serious concern because it could dilute any survival benefit. The real issue is that finasteride is associated with 350 fewer cases of Gleason 2-6 prostate cancer, six more cases of Gleason 7, and 37 more cases of Gleason 8-10. How do you balance that out? I must admit, I don’t know.
A WHO-NCI-American Cancer Society panel came together and stated that grade should not be used after therapy. In fact, we’ve been criticized for publishing Gleason scores in the study because we were using a hormonal agent.
|
| |
|
|
| 4.14 |
|
| |
|
SLIDES 4.12 – 4.14 What do you do when the intervention may affect the marker for prognosis? The answer is you have to use another validated prognostic marker, but one doesn’t exist. Several thousand potential prognostic factors are available in prostate cancer, but none are validated.
The other discussion that often arises is, “This group is at low risk and had PSA levels less than 3 ng/mL at the outset. Obviously, the tumors prevented have no clinical importance.” However, patients in practices with the same grade of prostate cancer are often offered radical prostatectomy.
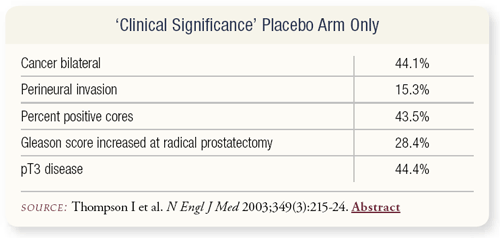
In terms of clinical significance, these are preliminary data. In the placebo arm only — which includes a mix of biopsy and radical prostatectomy — bilateral disease occurred in 44 percent, perineural invasion in 15 percent, positive cores in 43 percent.
Gleason score increased at radical prostatectomy in 28 percent of patients, and pT3 disease occurred in 44 percent. This pattern closely mimics the type of disease we would consider to be clinically or biologically consequential.
|
| |
|
|
| 4.15 |
|
| |
|
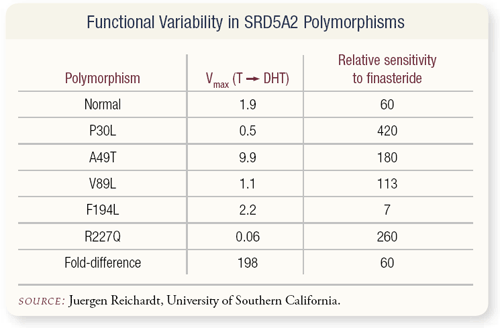
SLIDE 4.15 Where are we going in the future? We know the hypothesis that finasteride competitively inhibits 5-alpha-reductase type 2. Of interest, the gene that codes for that has a number of polymorphisms that code for variance of the enzyme. They have different abilities to make dihydrotestosterone (DHT) and different sensitivity to finasteride.
It may very well be, for example, from the least to most active in combination, there is a 300-fold variation in the activity of finasteride. If you’ve ever wondered why an individual comes in to be treated for benign prostatic hyperplasia (BPH) and has an immediate response, it is perhaps related to this phenomenon, although that’s never been investigated. It’s just a hypothesis that we’re investigating as part of a program project grant.
|
| |
|
|
| 4.16 |
|
| |
|

SLIDE 4.16 The more compelling rationale for the prevention of prostate cancer has to do with all the issues discussed here, because I have very serious concerns that our early detection efforts may be off the mark.
For example, based on Medicare data in men who have prostate cancer and undergo treatment, approximately 30 percent or more will require adjuvant therapy within five years. These are patients with rising PSAs, seminal vesicle invasion, positive margins and so forth. Using SEER data from 1976 to 2000, one could argue that mortality has not changed much in the United States and yet our diagnostic rate has exploded.
|
| |
|
|
| 4.17 |
|
| |
|
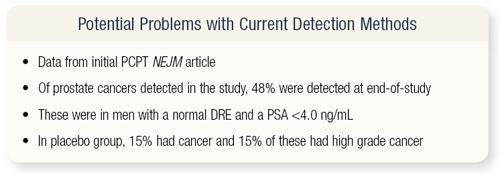
SLIDE 4.17 According to the original publication from last year, of the patients with prostate cancer detected in the study, about 48 percent were detected at the end of the study and were in men with a normal digital rectal exam (DRE) and a normal PSA, or at least a PSA less than 4 ng/mL. In the placebo group, approximately 15 percent of these men had cancer, and about 15 percent had high-grade disease.
|
| |
|
|
Select publications
|
|
|
|
|
|
|
|
|
|