 Home: PCU
3|2003: Laurence Klotz, MD, FRCSC Home: PCU
3|2003: Laurence Klotz, MD, FRCSC
 |
|
Laurence
Klotz, MD, FRCSC |
| |
| |
Professor, Department of Surgery, University of Toronto
Chief, Division of Urology, Sunnybrook Health Science Centre
Research Director, Division of Surgical Oncology, Toronto
Bayview Regional Cancer Centre
Founder, Prostate Cancer Research Foundation of Canada
Founder and Chairman, Canadian Urology Research
Consortium
Chair, Canadian Uro-Oncology Group
Chair, Global Genito-Urinary Oncology Group |
Edited comments by Dr Klotz
Clinical benefit of maximum androgen blockade
I’m a moderate supporter of maximum androgen blockade,
and I believe the pendulum has swung back too far against this
approach. There are 27 prospective randomized trials, involving
roughly 6,000 patients, all started in the 1980s. The mortality
data show a minor survival benefit — about a 10 percent relative
improvement and 3 percent absolute improvement at five years. In
2000, the group at Hopkins published an influential article entitled, “Complete
androgen blockade for prostate cancer: What went wrong?” The
article implies that we were mistaken, but to me, it’s all
about prolonging survival so I don’t dismiss the minor survival
benefit.
On the other hand, maximum androgen block (MAB) adversely impacts
quality of life and it’s costly, so one has to decide if
it’s worth it from a clinical perspective. When calculating
the cost based on a three-month survival benefit, the cost per
month of improved survival is actually quite reasonable compared
to chemotherapy for lung cancer or hormone-refractory breast cancer.
I believe patients in whom survival is the primary goal should
be offered total androgen blockade with the understanding that
there may be a modest effect on their quality of life.
Mechanisms of action of MAB and antiandrogens
It is believed that maximum androgen blockade blocks the adrenal
androgens, but I think the mechanism of action is more likely inhibition
of ligandindependent activation of the androgen receptor. There
is evidence that EGF, IL6 and a whole slew of cytokines activate
the androgen receptor in the absence of androgens and stimulate
mitosis. Antiandrogens block this. There are significant levels
of androgens from the adrenals — even in men on LHRH agonists — so
it may be that both mechanisms are at work, but there’s not
a lot of evidence that adrenal androgens have much impact on cell
growth.
In hormone-refractory prostate cancer, an antiandrogen works to
some degree, and it can also work as an androgen receptor agonist — you
obtain both effects. That explains the antiandrogen withdrawal
effect where, if a patient is progressing on total androgen blockage
and you stop the antiandrogen, there is a response. It acts as
an agonist because you have androgen receptor mutations that reverse
the effect of the antiandrogen, but in the nonhormone-resistant
patient, it acts as an inhibitor.
High-dose bicalutamide therapy in the adjuvant
setting
Bicalutamide 150 mg has just been approved in Canada for use
in patients who’ve elected watchful waiting, which is not
the indication for much hormone therapy, but at least it’s
now available. I think there’s a role for bicalutamide in
high-risk patients when it’s used in the adjuvant setting
after surgery, based on the Early Prostate Cancer (EPC) trials.
The data showed a substantial difference in the time to a development
of bone metastases in this subset of patients treated with two
years of adjuvant bicalutamide. While the difference is not as
impressive in the overall group, the EPC demonstrated an important
benefit of adjuvant therapy to this subset of patients and no study
had done that before.
Bicalutamide: An androgen receptor antagonist
Bicalutamide is a superior antiandrogen — not only because
it has fewer side effects and it’s administered only once
a day — but it’s also a more effective androgen receptor
antagonist.
Antiandrogen implies the competitive blocking of the binding
of dihydrotestosterone to the androgen receptor, but antiandrogens
also act to a large degree as androgen receptor antagonists. In
the absence of androgens, which is when they’re mostly used,
they block androgen receptor activation by nonliganddependent mechanisms.
Bicalutamide does that much more effectively than the other antiandrogens,
but it wasn’t around when all these randomized trials were
conducted. It’s possible that the survival benefit with bicalutamide
is significantly greater than has been demonstrated with the other
nonsteroidal antiandrogens.
Bicalutamide monotherapy versus LHRH agonists
Data from randomized trials in metastatic disease comparing bicalutamide
to an LHRH agonist show a six-week difference in survival favoring
the LHRH agonist. Efficacy includes long-term side effects and
quality of life, and in the average 70- year-old being treated
with hormonal therapy for a rising PSA, a minor difference in survival
is not the issue.
When compared to LHRH agonists, monotherapy with bicalutamide
has quality-oflife benefits that make it a very attractive therapy.
In addition to patients having an increased likelihood of retaining
sexual function, there is evidence that bicalutamide stabilizes
bone mineral density. While it is not conclusive, the bone density
data is very encouraging because we are treating patients early
and many have a 15-year median life expectancy, so osteoporosis
can become a major concern.
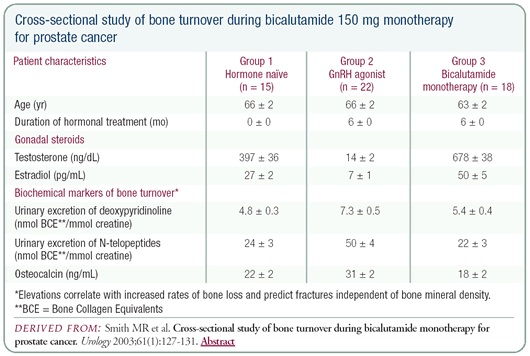
The downside of bicalutamide is gynecomastia — at least
half of the patients complain of significant gynecomastia, nipple
tenderness and pain — but there are a number of strategies
likely to block that. The traditional one is local radiation to
the breast and there’s also an ongoing study with tamoxifen.
Phase II trial of intermittent androgen suppression
I was a principal investigator on a Phase II trial in Canada
with 100 patients with PSAs greater than 6 ng/mL. The patients
received eight months of androgen suppression, followed by discontinuation
of therapy, and then resumption when their PSA was either greater
than 10 ng/mL or greater than their pretreatment level, whichever
was lower. This trial, as well as others, showed that intermittent
therapy resulted in patients being off treatment about 50 percent
of the time, which has significant quality of life benefits. And,
based on historical controls, there doesn’t seem to be any
adverse effect on the median survival.
In this study, quality of life domains, including sexual function,
generally returned to baseline three to four months after discontinuing
therapy. The majority of patients had relatively prompt recovery
of testosterone levels — 50 percent of patients had normal
levels by four months and 35 percent recovered to just subnormal
levels. In 15 percent of these men, levels remained castrate so
one criticism of this approach is that, in spite of stopping therapy,
not all patients are re-exposed to testosterone.
SWOG-JPR7: Phase III randomized study of intermittent
versus continuous androgen suppression
This study is supported by the NCI Clinical Trials Support Unit,
which means any member of any cooperative group trial in the United
States or Canada can enter a patient in the trial. The eligibility
criteria require that patients have received radiation therapy,
either as primary or salvage treatment, have a rising PSA of 3
ng/mL or greater and have no evidence of metastatic disease.
Patients are randomized between intermittent and continuous androgen
suppression. The intermittent therapy consists of eight months
of a LHRH analog and an antiandrogen for at least a month. Then,
if their PSA drops to normal during treatment, they stop treatment
and their PSA and testosterone levels are monitored every two months.
When the PSA returns to their pretreatment level or 10 ng/mL — whichever
is lower — they go back on therapy for another eight months.
The continuous therapy consists of a LHRH analog with at least
a month of antiandrogen therapy or a bilateral orchiectomy and
an antiandrogen.
The primary endpoint is the time it takes for the patient to
have a rising PSA in the face of castrate levels of testosterone.
We’re also looking at survival and quality of life endpoints.

Intermittent androgen suppression in clinical
practice
I support the current Phase III trial randomizing patients between
intermittent and continuous androgen suppression, so I don’t
promote intermittent hormonal therapy off-protocol. I explain to
patients that it’s investigational and we don’t know
what impact the unstable hormonal milieu will have on survival.
However, if a patient says, “Okay, I don’t want to
be randomized, I’ll just go on the hormonal therapy,” and
then eight months later, he wants to go off treatment, I don’t
argue as long as they understand it’s investigational.
When they understand the issues, approximately 95 percent of my
patients opt for intermittent therapy. This approach has received
a good deal of positive media attention in Canada, which may be
one reason why it’s widely accepted. Patients who’ve
experienced the side effects of androgen ablation therapy love
having a break from treatment. On the other hand, some patients
who have a strong PSA response to hormonal therapy don’t
want to tamper with their treatment. With intermittent therapy,
some patients become overly concerned when their PSA begins to
rise, but they have to understand that this is expected.
Management of the patient at high risk for progression
after surgery
If a patient is at high risk for progression after surgery — for
example, a positive surgical margin — one has to decide whether
adjuvant therapy is indicated. The difficulty is that positive
surgical margins are compatible with cure in about 50 percent of
patients, particularly if they’re micro-focal. My approach
is not to treat those patients with adjuvant therapy, but rather
to wait for a rise in PSA and then treat, based on the interval
between surgery and the rise.
If the PSA begins to rise, there are several protocols available,
such as the RTOG protocol of radiation therapy plus hormones versus
radiation alone versus hormones alone. Off-protocol, if it has
taken more than a year for the PSA to rise, I treat with radiation.
The data shows that almost all patients in whom the PSA never went
to undetectable levels — or began to rise within the first
year — have occult systemic disease, so I treat them with
androgen ablation therapy.
Next page
|