 Home: PCU
3|2003: Paul F Schellhammer, MD Home: PCU
3|2003: Paul F Schellhammer, MD
 |
|
Paul
F Schellhammer, MD |
| |
| |
Program
Director, Virginia Prostate Center
Professor of Urology, Eastern Virginia Medical School and Sentara
Cancer Institute
Trustee, American Board of Urology |
Edited comments by Dr Schellhammer
Anxiety caused by discontinuing hormonal therapy
When my PSA became elevated after the radical prostatectomy,
I was treated with radiation therapy and six months of combined
androgen blockade — goserelin and bicalutamide. I am now
in PSA remission, have discontinued those therapies and have been
off hormonal therapy for three months.
I recognized that while taking therapy is a nuisance, it’s
also very comforting because you are doing something active. When
I discontinued the therapy, I was relieved to be finished with
the burden, but I realized that the antitumor effect of therapy
was truly being tested for permanence and durability. Stopping
therapy has resulted in some subclinical anxiety. While I’ve
read about this in the past, I truly appreciate it now. Part of
me says, “Gosh, why stop what’s working? My ‘crutch’ is
being taken away.”
Tolerability and side effects of combined androgen
blockade
I knew what the side effects were and I anticipated them. I had
no breast effects whatsoever — no tenderness, no enlargement.
I didn’t have any mood swings or depression, and my wife
didn’t comment about any changes in my personality. The most
dramatic change was fatigue — the feeling of not having the
extra drive to accomplish things. I experienced a lack of vigor
and vitality, which crept up on me very insidiously. I hardly knew
it was happening. I was just more tired and looked forward to that
mid-day nap and got to bed a little earlier. I play tennis and
playing a set became much more of an effort.
Unfortunately, my thinking also became slower. As we age, we
all lose some of our recall ability for places, names and facts,
but this seemed to be an abrupt step down. I didn’t anticipate
it, but when it happened I said, “Well, I hope it is due
to the therapy, which is reversible, rather than a lack of cerebral
blood flow.” I haven’t been off therapy long enough
to know. My testosterone is just barely starting to make its way
up past the median castrate range, so I suppose I’m not physiologically
recovered.
I had frequent hot flashes, which were bothersome. They weren’t
drenching sweats, but rather the kind of reaction one might have
going into a tough exam or giving a talk — sweaty palms and
feeling warm. Rarely did they wake me up at night. They were more
annoying than incapacitating, like they are for some people. I
experienced an amazing diminution of libido. It’s a remarkable
and dramatic transformation that I only really discussed with my
wife and one or two other friends who are urologists. The feelings
of tenderness and the handholding or kissing certainly weren’t
impacted in any way, but the arousal that often goes along with
that — in the form of an erection or the feeling of a pelvic
rush — wasn’t there.
As a physician and scientist, it’s interesting to experience
what you’ve read about. You not only know what’s happening,
but you can feel it transpiring within your own body — like
an internal experiment.
Duration of androgen deprivation
The information regarding androgen deprivation and salvage radiation
therapy is very scant, and it’s all a transferal from the
clinical trials of primary therapy that showed a benefit from combining
the two therapies. The optimal duration of therapy is unclear.
We have information about two and three years of therapy but not
about lesser duration. Several salvage protocols have used six
months. Dr D’Amico was evaluating six months as primary therapy,
and it seemed long enough but not too long, so I arbitrarily chose
that as a convenient duration of therapy. I began external beam
radiation therapy about one month after initiating androgen deprivation
and continued the hormonal therapy for six months.
Personal experience with salvage radiation therapy
When recommending salvage radiation therapy for a rising PSA,
I tell patients that I’ve approached the issue both scientifically
and personally. The literature indicates that a minority of radiation
oncologists currently recommend it. Combining androgen deprivation
with radiation adds incremental benefit and is a reasonable choice,
and that’s the treatment I decided upon.
I experienced more radiation-induced proctitis than either the
radiation oncologist or I anticipated. It was so intense during
the last two weeks of treatment that we decided to reduce the dose
to less than 65 Gy. The proctitis lasted for about three months.
Now, when I counsel patients about radiation therapy, I have to
put this experience aside, because it’s not consistent with
what others have reported to me. My tissues must have been particularly
sensitive.
Impact of personal experience with prostate
cancer on counseling patients
I believe there’s a tendency for physicians — given
the repetitive nature of their recommendations — to lose
the sense of morbidity that may accompany treatment and the potential
variability between patients. Sometimes we are impressed that for
certain patients hot flashes are overwhelming and for others they
are not. Sometimes we may say, “Well, it might be an internal
reaction to the same event,” but that event certainly is
different and it’s not only perception, it’s reality.
I live in a small community and news travels quickly, so patients
will often ask me to relate to them my experiences with prostate
cancer treatment. I do this only after discussing their treatment
options in detail, because I don’t want to immediately introduce
biases into their decision-making.
Anxiety and follow-up PSA testing
I have a positive outlook on the future, but the PSA test is
a constant reminder of my situation. I am apprehensive before the
test and before opening the envelope with the test results. It’s
a tremendous relief when my PSA has not risen, even though it’s
only for another two, three or four months until the next PSA is
done.
I presume that over time — with a good outcome and a low
PSA — my anxiety will lessen. However, after a period of
time with a normal or undetectable PSA, if a patient has a biochemical
relapse, the whole scenario will be relived once again. I’ve
thought that prostate cancer mimics life in its slow and steady
attrition. You don’t receive the cure label and you’re
constantly impressed by the fact that this underlying issue remains.
Early versus deferred hormonal therapy
I’ve been intrigued by the back and forth discussions about
giving androgen deprivation early versus waiting until clinical
failure occurs, which has been the defensive position of urologists.
There are several trials that have looked at this issue and the
one that rings strongest in most urologists’ minds is the
VA trial of 30 or 40 years ago, which didn’t really demonstrate
a benefit for earlier administration of DES. However, the patients
in that study were much different than those we see today. They
had a huge disease burden, with either a large primary or metastatic
disease. It may not be appropriate to generalize the results of
that study to our current group of patients who have minimal disease.
The Medical Research Council trial attempted to evaluate the
issue of earlier versus later treatment, and they demonstrated
a clear diminution in morbidity from the disease if androgen deprivation
was administered at the time of diagnosis versus at some later
time, either precipitated by symptoms or clinical progression.
So, you could minimize urethral obstruction, vesicle outlet obstruction,
skeletal events, paraplegia, spinal cord compression and so forth,
all of which I think are worthwhile. They also showed a survival
benefit in M0 disease. Now, as time goes on, that survival benefit
becomes less obvious. If you’re instituting a therapy in
men who are in their late 60s or 70s and you follow them for 10
to 12 years, there are enough deaths from causes other than prostate
cancer that will bring the curves together.
We have always treated prostate cancer relatively late with hormonal
therapy. There is evidence that the best time to treat any cancer
with any pharmacological agent is earlier rather than later. The
entire rationale for adjuvant therapy is that mutations accumulate
with time and if the volume of the disease is least and therefore
the mutational capacity is least, then there is perhaps a window
in which therapy could be curative. I know that sounds almost completely
anathema to the thinking of urologists with regard to prostate
cancer, but the test has never been applied early enough. In urology
we say, “Well, it’s good palliative therapy,” which
it is, but I think the mistake is that we say that’s all
it could be.
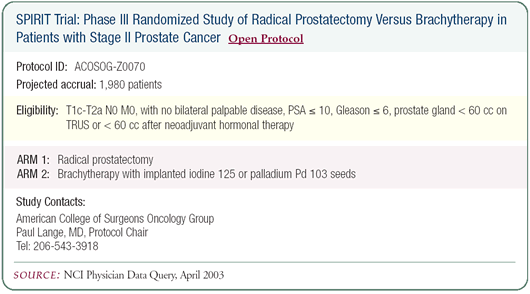
Update on the SPIRIT trial: Radical prostatectomy
versus brachytherapy
Accrual to the SPIRIT trial has been discouragingly slow. Our
center has randomized one patient out of 25 to whom the trial was
presented. I’m not being overly pessimistic, but I’d
be dramatically encouraged if I presented it to 25 people and 10
accepted. Other physicians have told me that they presented the
trial to even more patients with a worse rate of acceptance, so
the ratio might be a lot lower than one in 25. Patients recognize
the importance of the trial and are very appreciative of what we’re
doing. I think that relinquishing control over their treatment
decision-making is the overriding reason for choosing not to participate.
The Canadians have enrolled about five times as many patients
to the trial as we have in the United States, which is a real tribute
to their dedication to clinical trials. Another potential reason
for the lower accrual rates in the United States is that our patients
may not be simultaneously receiving an explanation of the risks
and benefits of radiation therapy and prostatectomy. Once a patient
has been directed toward one therapy, it’s difficult to go
back to “square one” and consider other options.
Select publications
|