 Home: PCU
3|2003: Anthony L Zietman, MD, FRCR Home: PCU
3|2003: Anthony L Zietman, MD, FRCR
 |
|
Anthony
L Zietman, MD, FRCR |
| |
| |
Associate Professor, Department of Radiation Oncology,
Harvard Medical School
Director, Residency Training for Radiation Oncology,
Department of Radiation Oncology, Genito-Urinary
Oncology Unit, Massachusetts General Hospital
|
Edited comments by Dr Zietman
Impact of PSA screening on prostate cancer detection
With the advent of the PSA screening revolution, one would expect
the median age at which we detect prostate cancer to be decreasing,
but that is not the case. The median has remained the same, but
the Gaussian peak is much more spread. We are detecting patients
much younger with early disease, but we are also detecting an increasing
number of older patients. These older men are the patients who
perhaps don’t need any treatment other than watchful waiting.
Watchful waiting in elderly patients
Over the past few years I’ve become very interested in
the question, “Who really needs treatment?” Since about
1993 I’ve been recommending watchful waiting to elderly patients
with small volume, low-grade disease. Paul Schellhammer and I recently
reported on a couple of hundred patients with prostate cancer in
their early 70s, each with a comorbid condition, who did not receive
radical treatment.
It was striking to me that only one or two patients actually
died of prostate cancer, but that so many patients found watchful
waiting very difficult. Despite their doctors reassurance that
prostate cancer was the least of their medical worries, approximately
50 percent of the patients elected treatment within five years,
even though they had almost no signs of progression. So while watchful
waiting is probably the right thing for an enormous number of elderly
patients with early prostate cancer, it is a very difficult thing
to do.
Watchful waiting in younger patients
Watchful waiting in younger men may be safe to do, but if a patient
has a life expectancy of 20 or 30 years, you’re probably
only delaying treatment. It may be desirable to do so for one or
two years while they deal with other big issues in their lives — maybe
they plan to retire in a year and want to deal with treatment then.
You’re only deferring the inevitable — it may be 5
or 10 years or more until they need to be treated, but I believe
eventually they will need to be treated.
Efficacy of watchful waiting versus radical
prostatectomy
There was an important trial reported in the New England Journal
of Medicine in which one-half of the patients were managed expectantly
and half received immediate radical prostatectomy. After eight
years they found only a very small disease-specific survival advantage
in patients who underwent radical prostatectomy rather than watchful
waiting.
The trial involved Scandinavian patients — most of whom
had palpable disease — who were not screen-detected and had
a median PSA of around 10 ng/mL at the time of diagnosis. If we
try to translate that to American patients, they are, on average,
diagnosed by PSA approximately seven years before this point, so
that eight-year data from the Scandinavian study could probably
be translated to 15-year data for a US population. I think the
difference between the two arms in the Scandinavian trial will
diverge with time, but they haven’t yet. As a result when
I look at our patients, I think about that small survival advantage
and their life expectancy and wonder how much we are actually gaining
when we radically treat them.
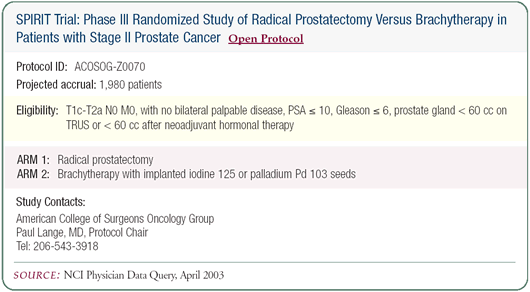
Radical prostatectomy versus radiation therapy
in younger patients
Currently in my practice, for patients in their 40s and early
50s, I recommend radical prostatectomy rather than radiation therapy.
It’s not that I can prove prostatectomy is superior, rather
I just wonder what a younger man is doing with prostate cancer.
Is his entire prostatic epithelium in some way genetically dysfunctional
such that if I treat him with radiation, he will go on to develop
a new cancer in 10 or 20 years? I don’t know the answer to
that question. Also, I think younger men benefit from the prognostic
information that’s obtained at the prostatectomy.
Conformal radiation therapy
Phase III trials have shown an advantage to conformal over conventional
radiation, so most of us use conformal therapy. There are new types
of conformal radiation — proton beams — intensity-modulated
radiation — that reflect technical advances that make treatment
a bit more accurate. Whether that slightly increased accuracy translates
into reduced morbidity or an increased cure rate, nobody knows
yet. It has certainly increased cost of therapy. Whereas external
beam radiation used to be comparable to radical prostatectomy in
terms of cost, it is now much more expensive.
At this time, conformal therapy is almost entirely CT-scan-based.
Some institutions are using MR-based planning and I believe functional
MR will someday have a role with brachytherapy and perhaps external
beam radiation. The idea of functional MR is to identify a dominant
tumor focus and give that focus extra radiation. In the next decade
I think we’re going to see an enormous investigative push
in this area. Whether it will be fruitful or not, I don’t
know.
Quality control in the delivery of radiation
therapy
In terms of quality control, external beam therapy is delivered
fairly well nationally. There is a national quality control body
that compares academic centers with community centers and reports
their findings every five years. The only substantial difference
reported is that academic centers are bolder with external beam
therapy. They use their conformal radiation to treat at higher
radiation doses, which probably translates to an increased number
of cures. That does not mean that radiation therapy in community
practice is delivered badly, it’s just delivered timidly.
As for brachytherapy, our patterns of care survey in 1994 revealed
that 3 percent of early prostate patients were treated with brachytherapy,
which increased to 27 percent by 1999 and I suspect it’s
50 percent by now. We didn’t have enough data from the 1994
and 1999 surveys to really draw conclusions, but I think quality
control nationally is very loose. The American Brachytherapy Society
is trying to develop quality control guidelines, but it’s
proving a difficult task.
Proficiency in brachytherapy
Radiation oncologists perform brachytherapy at many sites. We
understand it well and we know its pitfalls. In the community,
prostate brachytherapy is becoming a urologic procedure with radiation
oncology backup rather than the other way around, and urologists
are not as well-versed in the procedure. Legally, a radiation oncologist
has to be involved in brachytherapy, but I think many radiation
oncologists are abdicating their responsibility. They may be present
at the procedure or they may simply be involved in the planning
or they may only write the prescription.
Brachytherapy is operator-driven and has a long, slow learning
curve. Suboptimal results are usually due to inexperience and most
urologists are only in their first one or two years of performing
the procedure. The problem that usually occurs is that the seeds
are misplaced, resulting in underimplantation of the target area.
The seeds end up somewhere else, such as in the perineum or around
the sphincter, with uncertain consequences. We haven’t had
time to document the patient outcomes, but we presume they will
be worse.
External beam versus brachytherapy: Side-effect
profiles
For men in their late 50s and older, selecting between brachytherapy
and external beam therapy is not based on superiorit, but rather
choosing between morbidities, and that’s up to the patient.
Short-term, external beam therapy patients receive a mild, acute
radiation prostatitis, a mild cystitis that manifests as urinary
frequency and urgency, and they may experience proctitis, but it’s
generally minor. Brachytherapy patients experience an immediate,
traumatic prostatitis with the insertion of the needles and then,
about 10 to 14 days later, they experience edema of the prostate
and urinary frequency and urgency. By three months, the side effects
diminish and you can’t really distinguish external beam patients
from brachytherapy patients; however, there is no doubt that short-term,
external beam patients have fewer problems.
Long-term effects of radiation therapy may include proctitis
or persistent urinary symptoms, but the primary problem is erectile
dysfunction. I think radiation oncologists and surgeons alike overstate
the potency of our patients. When independent investigators have
prospectively evaluated patients being treated with either brachytherapy
or external beam radiation they find that in excess of 50 percent
lose their erectile function. The risk of erectile dysfunction
is even greater after surgery. Although there may be individual
surgeons for whom it is less, if you look at national statistics,
only about one-third of surgical patients retain useful erectile
function without assistance.
Next page
|