 Home: PCU
3|2003: Anthony L Zietman, MD, FRCR Home: PCU
3|2003: Anthony L Zietman, MD, FRCR
Combination brachytherapy and external beam
radiation
There’s been a movement nationally to add external beam
radiation to brachytherapy. The combination is very expensive with
significant side effects, so we need to determine if there is a
cancer advantage sufficient to warrant such heavy-duty treatment
for low- and intermediate-risk disease.
The RTOG is about to open a trial looking at brachytherapy with
or without external beam radiation. For the first part of the trial,
we’re looking for patients with intermediate-risk disease — PSAs
greater than 10 ng/mL and Gleason scores of 7 — who might
benefit from the addition of external beam therapy. The treatment
will either be full-dose seed implant, palladium or iodine, or
a reduced-dose implant together with 45 Gy external beam radiation.
Androgen deprivation will not be allowed in the trial to avoid
confounding issues. Triple approach therapy including androgen
deprivation
An enormous number of patients are receiving off-protocol, triple
therapy consisting of external beam radiation, brachytherapy and
androgen deprivation. I don’t use triple therapy because
I tend not to use hormone therapy with brachytherapy. We know from
mouse experiments, in vitro experiments and clinical trials that
hormone therapy, when added to external beam radiation, pulse high-dose
radiation, is synergistic. But the method of cell killing by lowdose-
rate brachytherapy is completely different. It is entirely possible
that hormone therapy could actually reduce the cell killing by
taking cells out of cycle. When the Seattle Group analyzed their
large series of patients who had received prostate brachytherapy
and stratified them by risk, they found that, in the intermediate-risk
group, patients who had been given some hormone therapy first,
did worse than those who’d had brachytherapy alone.
Neoadjuvant and adjuvant hormone therapy in
combination with external beam radiation
Several randomized trials from the 1980s, published in the 1990s,
showed that patients with intermediate-risk disease did better
in terms of local control and disease-specific survival if they
received neoadjuvant hormone therapy, which consisted of hormone
therapy before and during radiation. That then became the standard
of care. We’re not sure whether hormone therapy and external
beam radiation are additive or synergistic (they appear to be synergistic
in the mouse model), and it might be that local control is substantially
improved by the addition of hormone therapy.
The RTOG has conducted trials comparing external beam radiation
with a short course versus a long course (two or three years) of
adjuvant hormone therapy. For patients with high-risk features,
Gleason 8, 9 and 10, there is a small but significant survival
advantage eight years later for the longer course of hormone therapy.
Hormonal therapy based on risk
In our patterns of care survey we find most patients with high-risk
and intermediate-risk prostate cancer are receiving hormone therapy,
as well as 50 percent of early-stage patients. It has just become
practice without evidence. In my practice, I don’t use hormone
therapy for patients at low risk. For patients at intermediate
risk, I recommend neoadjuvant hormone therapy; for those at high
risk, I use long-term adjuvant androgen deprivation.
No evidence supports the need for hormone therapy in patients
at low risk (PSA less than 10 ng/mL, Gleason 6 or less). A randomized
RTOG trial with 1,600 patients was completed several years ago.
It doesn’t plan to report for many years, but as far as we
know now, radiation alone appears to be sufficient for men with
low-risk disease.
For intermediate-risk disease (Gleason 7, a bulkier tumor, PSA
of 10 to 20 ng/mL) we normally add neoadjuvant and concurrent hormone
therapy. We’re conducting trials to determine what duration
of neoadjuvant hormone therapy is the most efficacious. We found
in animal models that you obtain the most benefit from the radiation
treatment if you give it at the point when you have the maximal
response to hormone therapy. That’s why we’re testing
six months versus the current standard of two months of hormonal
therapy, because we don’t see much shrinkage after only two
months. Off protocol, I give patients the single LHRH agonist injection
at three months and plan for radiation three months later.
For patients with high-risk disease (Gleason 8, 9 and 10 or a
lower Gleason grade with a PSA greater than 20 ng/mL) we generally
use postradiation, adjuvant LHRH agonists for two or three years.
European and American trials suggest two or three years is better
than a short course, but whether two or three years is better,
we don’t know. In my practice, I generally review the situation
after a year and if the patients are truly miserable on their LHRH
agonist, as many of them are, I may stop then or I may just give
a second year of therapy rather than a full three.
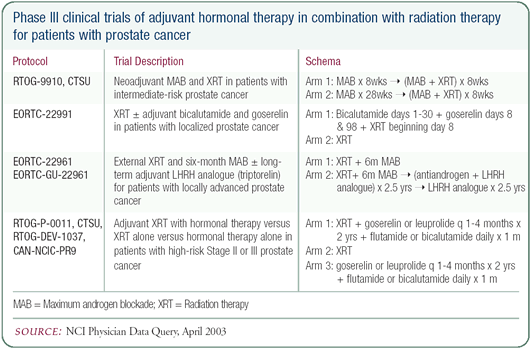
Hormonal therapy for systemic versus local disease
The primary intent of using postradiation, adjuvant hormonal
therapy in patients with high-risk disease is to deal with micrometastatic
disease. Interestingly, if those same patients underwent surgery
rather than radiation for local therapy, they probably wouldn’t
receive adjuvant androgen deprivation therapy. In radiation, we
have two randomized trials that have shown a survival advantage
with the combination, so it’s become the standard of care.
It may be that if those same patients went into a randomized trial
of hormonal therapy following a radical prostatectomy, rather than
radiation, that the results would also be positive and the practice
patterns would change. In dealing with prostate cancer, we need
to recognize that we are dealing with a systemic disease.
Toxicity profile: Bicalutamide 150 mg versus
LHRH agonists
We’ve completed a randomized trial at Massachusetts General
Hospital in which we compared the effects of high-dose bicalutamide
to LHRH agonists with regard to body composition, bone mass and
a number of psychological and quality of life endpoints. Until
we analyze the data, it’s too early to say, but it appears
that bicalutamide has a less profound effect on body fat, less
fatigue and bone mass goes up, not down. Also, my guess is that
the quality of life will be better with high-dose bicalutamide
than with an LHRH agonist.
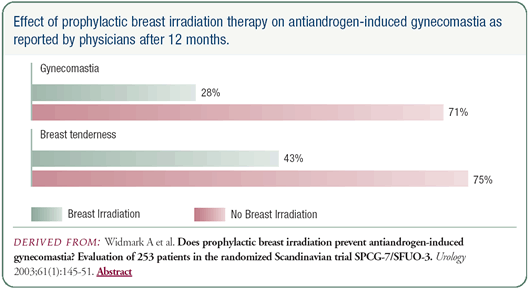
There are some downsides to high-dose bicalutamide. Gynecomastia
can affect 70 percent of men. However, we know from a Swedish trial
that if you administer 1 to 3 doses of radiation to the breast
prior to starting bicalutamide or flutamide, you can reduce the
risk of gynecomastia from approximately 70 percent to about 25
percent. It also reduces breast tenderness, although to a lesser
degree.
Long-term androgen deprivation and bisphosphonates
We conducted a study of men on long-term androgen deprivation
in which half were treated with pamidronate and half were not.
Bone density was measured at six months and one year. After one
year, for those who did not receive pamidronate, the median bone
loss was about 5 to 7 percent. It was substantially less, or not
at all, for those who did receive pamidronate. We didn’t
expect our trial to result in every patient receiving androgen
deprivation therapy to be treated with pamidronate or, by extension,
zoledronate, which is more convenient. And we don’t know
how clinically relevant the bone loss is and that still needs to
be studied. We simply showed that there is bone loss and it can
be prevented.
Salvage radiation
Despite the curative intent of radical prostatectomy, PSA goes
up after the procedure in 30 to 50 percent of patients, depending
on what series you review. So there’s no question that failure
is a problem after surgery, but where are the patients failing — locally
or at distant sites? In a large series from the University of California
at San Francisco, patients with a rising PSA after prostatectomy
had their prostate beds re-biopsied and approximately 50 percent
showed evidence of local failure. That would suggest that postoperative
radiation, or salvage radiation given when the PSA rises, might
cure some patients.
We, and many others, have looked extensively at the role of salvage
radiation and it appears it cures about 30 percent of these patients.
Why don’t we cure more than 30 percent? I think because,
even though the PSA comes down in 70 percent of those we treat,
obviously 40 percent have distant disease as well, so the PSA subsequently
rises.
We’re selective in whom we treat with salvage radiation.
If the PSA is going up rapidly, the original tumor was a Gleason
9 or 10 and there’s extensive seminal vesicle involvement
and positive lymph nodes, the chance of occult micrometastatic
disease is so high that salvage radiation is a waste of time. For
those patients, it’s a question of hormonal therapy, either
now or at some future time.
Combining hormone therapy and salvage radiation
The data is pretty thin with regard to combining hormone therapy
with salvage radiation. We know that when we treat patients with
even the standard dose of 64 Gy salvage radiation, subsequent local
recurrences are rare. So, radiation is at least getting the job
done in the tumor bed and hormone therapy would be added to eradicate
micrometastatic disease, for which we’d consider three years
of androgen deprivation.
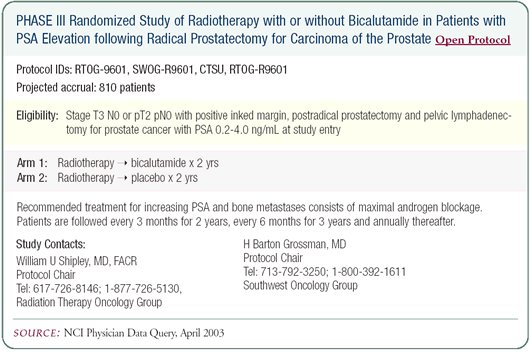
While one might presume that the effect of high-dose bicalutamide
on occult micrometastatic disease is the same as an LHRH agonist,
we don’t know for certain. The RTOG is conducting a trial
in which all patients receive salvage radiation and half receive
high-dose bicalutamide. About 900 of the projected 1,400 patients
have been entered, so we should finish enrollment this year. I
have patients enrolled in this study and, while it’s double-blind,
we can easily tell who is on the bicalutamide by the breast effects.
About three or four weeks after starting bicalutamide, patients
develop nipple tenderness. It takes a few months before they develop
gynecomastia. Hot flashes are not a problem with the bicalutamide.
Sexual dysfunction is not an immediate problem, as it is with an
LHRH agonist, but it’s still a problem. Even if a patient
still has erectile function after surgery, the radiation and high-dose
bicalutamide are a double assault and it’s very unusual for
patients to still have erectile function after two years.
Select publications
|