 Home: PCU 6|2003: Colleen A Lawton, MD, FACR Home: PCU 6|2003: Colleen A Lawton, MD, FACR
 |
|
Colleen A Lawton, MD, FACR |
| |
| |
Professor of Radiation Oncology,
Medical College of Wisconsin
Milwaukee, Wisconsin |
Edited comments by Dr Lawton
RTOG-85-31: Radiation therapy and androgen deprivation for locally advanced prostate cancer
Trial design and efficacy data
RTOG-85-31 was a prospective, randomized trial for patients with locally advanced disease. The trial began in 1985 and compared radiation alone to radiation plus indefinite hormone manipulation in the form of an LHRH agonist. Patients on the radiation-only arm received an LHRH agonist at the time of failure - generally biochemical failure.
When the results of RTOG-85-31 were first published, a significant benefit was seen in local control, distant disease and biochemical control for patients receiving adjuvant hormone therapy. We believed we would eventually see a survival advantage, but we did not see it at that early analysis.
The long-term data for RTOG-85-31 was presented at the 2003 ASTRO meeting and showed 10-year overall and cause-specific survival advantage for patients receiving adjuvant hormone therapy. I believe patients are more concerned about cause-specific survival than overall survival. Patients want to know if they are going to die of prostate cancer, not whether they are going to die of a heart disease or stroke in the interim.
Hormone therapy appears to do two things: It shrinks the volume of the cancer in the area, making it easier to radiate the prostate bed, and it treats micrometastases.
Dose escalation in radiation therapy
We are learning that for patients with locally advanced disease, we need to add hormones and to dose-escalate radiation therapy. The million-dollar question from RTOG-85-31 is whether the hormone therapy compensated for inadequate radiation therapy. Data suggests that we need to deliver higher doses of radiation than those administered in any of the RTOG trials, which was essentially a boost dose of 70 Gy to the isocenter, with the prostate itself receiving about 68 to 69 Gy. Probably the best prospective, randomized data regarding the need for dose escalation in patients with intermediate- and high-risk tumors comes from MD Anderson, but what advantage we obtain by adding that higher dose in addition to the hormones is still an unanswered question.
Osteoporosis and fractures while on long-term hormonal therapy
In RTOG-85-31, the length of hormone therapy was indefinite, so some of the patients are still on treatment 15 years later. Currently RTOG is developing a protocol to study the relationship between long-term hormone therapy and osteoporosis and fractures. We are hoping to accrue 700 patients within 12 to 18 months, and we expect to have an answer in the next two years. We will evaluate Dexascans to assess osteoporosis and T-spine films to evaluate the rate of fractures.
Multiple studies have demonstrated an increased rate of osteoporosis with long-term hormonal therapy, and I think this study will confirm that, but there's very little data on whether that translates into an increased fracture risk. There will be a quality-of-life instrument in this protocol, and I suspect we'll see more fractures and a decrement in the quality of life. If so, we need to determine what to do to avoid this toxicity for patients on long-term hormone therapy.
RTOG-86-10: Neoadjuvant androgen blockade for locally advanced disease
In RTOG-86-10, approximately 450 patients with locally advanced disease were randomly assigned to receive either no hormone treatment or total androgen suppression neoadjuvantly - two months prior to radiation therapy and then again during radiation versus radiation alone. Patients with lower Gleason Grades (six or less) benefited from radiation therapy.
One could postulate the hormone therapy shrank the lesions that weren't likely to metastasize, allowing the radiation to do its job. In contrast, the patients with high-grade tumors had too much micrometastatic disease and really needed long-term hormone therapy.
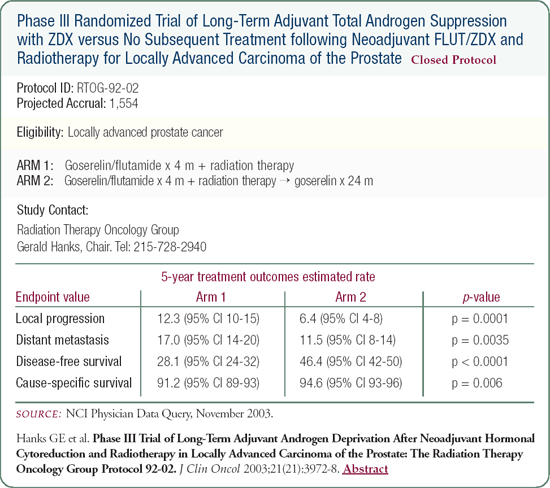
RTOG-92-02: Short-term versus long-term total androgen suppression for locally advanced disease
Patients in this trial received total androgen suppression in a neoadjuvant fashion, as in the treatment arm of RTOG-86-10, with or without two years of an adjuvant LHRH agonist. The data showed an advantage in local control, distant disease and biochemical-free survival for patients treated with long-term therapy, and a survival advantage in patients with high-grade disease. As the data matures, I believe we will see a cause-specific survival advantage as we did in RTOG-85-31.
Both trials RTOG-85-31 and 92-02 indicate, and it's generally accepted, that we need to include some degree of long-term hormone manipulation in treating patients with locally advanced disease, high-grade tumors and possibly patients with a high PSA.
RTOG-99-02: Adjuvant chemotherapy for patients with high-risk prostate cancer
RTOG has an ongoing trial looking at the value of chemotherapy in patients with aggressive disease - high PSAs, locally advanced disease and high-grade tumors. Patients are randomized between hormones and radiation versus hormones, radiation and chemotherapy.
It's an uphill battle to enter patients on study because of biases against chemotherapy, but we need to remember that just 15 years ago we thought there was no role for chemotherapy in non-small-cell lung cancer, and look at us today. I believe that slowly but surely we'll define a role for adjuvant chemotherapy, but it's going to take some time.

Case study: Patient presenting with locally advanced prostate cancer
History
This patient was in his mid-fifties when he had his first screening PSA, which was in the high forties. He was diagnosed with a T3, Gleason 7 (4+3), prostate cancer.
Follow-up
When I saw the patient, I recommended radiation therapy and androgen deprivation. I treated him with two months of neoadjuvant hormonal therapy to cytoreduce the tumor. Essentially, his therapy was the same as the long-term treatment arm in RTOG-92-02. He received bicalutamide and a LHRH agonist. The tumor responded nicely after two months and then we began radiation treatment.
I treated the nodes and pelvis, and to maximize his chance for survival, I used a higher boost dose to the prostate than in any of the RTOG studies. The patient tolerated treatment well and we will continue hormone therapy for two years. He's still receiving the LHRH agonist; his PSA is undetectable and he feels well.
Screening for prostate cancer
When this patient was diagnosed, he had a sense of anger that his doctor had never recommended PSA screening. It was his wife who suggested he should be screened during an annual exam. I believe it's unusual for patients to be well-screened for prostate cancer in the community.
Quality of life versus survival from the patient's perspective
I explained to this patient that when we use hormone therapy for such a long period of time, the likelihood is high that he will have erectile dysfunction. I do have a few patients who have regained sexual function after extended hormonal manipulation, but it's uncommon. Potency was a huge issue for this young patient, but survival was more important.
Patients have different goals. In this case, the disease was so advanced that the issue was survival. Patients at low risk realize they probably won't die of their disease, so quality of life becomes more important.
I didn't discuss the alternative of bicalutamide monotherapy with this patient, because I don't have data that shows it's as efficacious as an LHRH agonist and he was focused on survival. For the patient who wants to be treated with hormone therapy, but values quality of life as much as survival, I consider bicalutamide monotherapy.
"PSA bounce"
The biggest issue with "PSA bounce" is defining it. For the longest time we used the ASTRO definition for biochemical recurrence, which is three consecutive rises in PSA, but data from MD Anderson has demonstrated this probably isn't the best definition. If a patient has a rising PSA and then it goes down and subsequently rises two or three times, where do you count the first rise in PSA? It's difficult to determine, and we've all known patients with courses like that. Interesting new data suggests that an absolute rise of 2.0 ng/mL may be a better definition of recurrence, which is certainly a very clear definition.
Patients are so anxious about their PSA that they often want it tested during treatment; however, we can see fluctuations then, so I discourage testing. In the past, I checked the PSA on all my patients one month after treatment, but in five to 10 percent of cases, the PSA rose before it ultimately went down. That upset everyone, including me, so now I encourage patients to wait longer. With a patient like this, after the LHRH agonist is stopped, we may see a bounce at some point, and then hopefully a leveling off or maybe even a drop. That's something we carefully walk the patient through.
A father with locally advanced prostate cancer
Three and a half years ago when my father was almost 70 years old, he was diagnosed with a Gleason 7, locally advanced prostate cancer. His PSA was 93. He had not had routine screening but he was having some clinical symptoms. He was treated at my institution, and although I didn't treat him, I was in the background. He received radiation therapy and hormone therapy. At the outset, he received total androgen suppression with bicalutamide and an LHRH agonist, followed by long-term LHRH agonist therapy.
This experience certainly gave me a different perspective on being a doctor versus a daughter. There was a lot of tap dancing between those two roles when I spoke with him. My dad is a very traditional man, and he didn't know much about my work before that, but at the end of the day he respected what I did. It was difficult for my friends and family to believe he developed the disease - and in fact the very stage of the disease - I've spent my career investigating.
I'm a very religious person, as is my father, and I think that the good Lord had something to do with this. I had a chance to do something very meaningful for my father, not so much in instituting treatment, but in helping him cope with the side effects, obtain information and line up physicians. It brought us closer. I don't think it was an accident that this related to my life's work and that my father was among the men who benefited from it.
Select publications
|