 Home: PCU 6|2003: Gregory S Merrick, MD Home: PCU 6|2003: Gregory S Merrick, MD
 |
|
Gregory S Merrick, MD |
| |
| |
Medical Director, Schiffler Cancer Center, Wheeling Hospital
Associate Affiliate Assistant Professor of Physics
Wheeling Jesuit University
Adjunct Assistant Professor
The George Washington University Medical Center
Division of Radiation Oncology and Biophysics
Wheeling,West Virginia |
Edited Comments by Dr Merrick
Ongoing clinical trials of brachytherapy
Kent Wallner at the University of Washington and I are currently completing two brachytherapy trials. The first randomly assigns patients with low-risk prostate cancer (i.e., pretreatment PSA <10 ng/mL, Gleason score <= 6 and T1-T2 disease) to brachytherapy with either iodine 125 (I 125) or palladium 103 (Pd 103). The goal of this trial is to determine whether there is a difference in biochemical outcome or quality-of-life parameters (i.e., urinary, bowel and sexual function). The trial will eventually accrue 660 patients. Currently, around 600 patients are enrolled in that study.
The second trial is for patients whose disease has higher risk features (i.e., PSA = 10-20 ng/mL, Gleason score >=7 and T1-T2 disease). The standard of care for these patients has been five weeks of supplemental external beam radiation (45 Gy) followed by a palladium implant. In our series of patients, supplemental external beam radiation adversely affected long-term urinary function based on the Expanded Prostate Cancer Index Composite (EPIC) survey. External beam radiation can also increase the risk of rectal bleeding and erectile dysfunction.
Hence, the second trial in patients with higher-risk features will compare high and low doses of external beam radiation therapy followed by a brachytherapy implant. This trial will determine whether the dose of the external beam radiation can be reduced. It will have the same endpoints as the first trial: biochemical outcome and quality of life (i.e., urinary, bowel and sexual function). The trial will eventually accrue 680 patients; currently, around 600 patients are enrolled.
In the same patient population, RTOG recently opened a Phase III trial (RTOG-0232) comparing traditional external beam radiation therapy (45 Gy) plus an implant to an implant alone. RTOG-0232 will determine whether external beam radiation therapy is truly needed.
As we develop trials, we're looking for ways to further reduce morbidity, because we know the cure rates are very good. Early next year, we're hoping to begin a Phase III trial that will evaluate dose de-escalation in brachytherapy. Everything in radiation therapy - both for external beam and brachytherapy -has been about dose escalation. We think that with very careful intraoperative evaluation, we will be able to reduce the dose of the palladium implant by 10 to 15 percent without compromising cure, while further improving quality of life.
Hormonal therapy plus brachytherapy and external beam radiation
In patients with intermediate- or high-risk prostate cancer, a Phase III trial was going to compare six months of hormonal therapy combined with external beam radiation therapy and an implant to an implant and external beam radiation therapy alone. Unfortunately, that study accrued about 90 patients over three or four years and was closed.
At the present time, the only proven role for hormonal therapy with brachytherapy is to reduce the size of very large glands. Total androgen suppression is proven to shrink glands 35 to 45 percent. Some of our data in press will show that the response of the transition zone to hormonal therapy may be the best predictor of long-term urinary morbidity.
We do not have strong data to indicate that hormonal therapy alters biochemical outcome following brachytherapy. The Stock and Stone data show some suggestion for improvements; however, the follow-up in patients who are hormone-naïve has been significantly longer. Those curves will have to mature before making any definitive conclusions.
In our series, we have not found any improvement in biochemical outcome for patients with intermediate-risk disease treated with hormonal therapy. In patients with high-risk disease, we found a small but statistically significant improvement with the addition of hormonal therapy. The shortcomings of our data are the same as those of Stock and Stone's data: (1) it's not randomized, and (2) the follow-up of the patients who were hormone-naïve is significantlylonger.
Part of the problem is that we have tried to extrapolate the results of conventional doses of external beam radiation therapy with hormones to brachytherapy. Determining the role of hormonal therapy will require much better studies than we have done to date. A population of patients will probably benefit from hormonal therapy administered in combination with brachytherapy. This is a question that will be best answered in a prospective randomized trial.
Total androgen suppression
I always utilize an LHRH agonist in combination with an antiandrogen for cytoreduction. Data indicate that slightly more downsizing occurs with total androgen suppression - at least at three months - and we don't want to keep patients on hormones longer than necessary. In our series, we have demonstrated about 45 percent downsizing with an LHRH agonist and bicalutamide 50 mg per day.
When treating patients with external beam radiation therapy, I always start with total androgen suppression and usually continue the antiandrogen for four months. If the PSA is undetectable at four months, I stop the bicalutamide; if the PSA remains elevated, I continue it. After bicalutamide is discontinued, if the PSA creeps back up, I put them back on it.
Hormonal therapy after biochemical failure following brachytherapy
We manage patients with biochemical failure by instituting hormonal therapy when the PSA doubling time becomes less than 12 months, the PSA reaches an arbitrary level (e.g., 15 ng/mL), or both. In our series, the patients who failed frequently had very rapid PSA doubling times. We always begin with total androgen suppression. I continue the antiandrogen for four months and, if the PSA is undetectable, I consider discontinuing it. Some of those patients, however, are very nervous and say, "I don't want to quit taking the antiandrogen," and we certainly don't encourage them to quit.
Intermittent androgen suppression
I believe intermittent androgen suppression is a marvelous treatment approach for patients who are not overly sensitive to changes in PSA. We use it occasionally to treat patients with metastatic disease or biochemical failures. We usually treat with about nine to 12 months of hormonal therapy - I generally utilize an antiandrogen, bicalutamide, at least for the first four months. If the patient has a good PSA response, we stop the hormonal therapy and wait until the PSA approaches some arbitrary level. The quality of life for these men is very good. Patients are usually off of hormonal therapy for 12 to 18 months. When I reinstitute hormonal therapy, I always reinitiate bicalutamide.
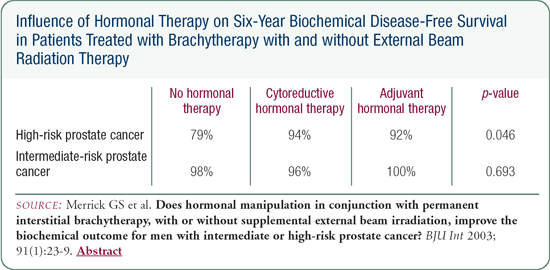
Short-term morbidities following brachytherapy
The usual short-term side effects associated with brachytherapy involve very intense prostatitis with symptoms of urgency, frequency, burning and nocturia. We demonstrated that the prophylactic use of alpha blockers, primarily tamsulosin, initiated before the implant and continued at least until the International Prostate Symptom Score (IPSS) was normalized, significantly lessened the irritative urinary symptoms and led to a more rapid return to the baseline IPSS. Short-term rectal morbidity following brachytherapy is minimal.
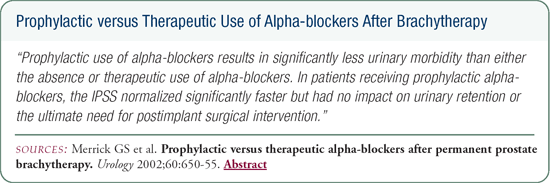
Long-term urinary outcomes following brachytherapy
We recently published a paper about the long-term urinary outcomes following brachytherapy as measured by the Expanded Prostate Cancer Index Composite (EPIC). We chose the EPIC instrument because it evaluates the irritative component that we're most concerned with following brachytherapy. Compared to a matched control group, the long-term EPIC scores were identical for the men treated with brachytherapy.
Surprisingly, patients treated with short-term (e.g., six months or less) hormonal therapy had slightly, though not statistically significant, better urinary function compared to men who did not receive hormones. However, men treated with hormones for more than six months had significantly poorer outcomes with regard to irritation and function. The strongest predictor of late urinary function was tobacco consumption. It affected all of the domains of the EPIC survey and the IPSS. The use of supplemental external beam radiation therapy also predicted long-term urinary morbidity; it had a deleterious effect on incontinence and function.
Long-term bowel function following brachytherapy
Brachytherapy also effects long-term bowel function. Using the Rectal Function Assessment Score (R-FAS), which is graded from zero (best function) to 27 (worst function), patients with newly diagnosed prostate cancer have a score of 1.6. Three years after brachytherapy, they have a score of approximately 4.2, and at eight years, they have a score of about 3.9.
Although there are changes in long-term bowel function following brachytherapy, they're relatively minor and may improve slightly with time. The best predictors of long-term morbidity following brachytherapy are the number of pretreatment bowel movements per day, the use of supplemental external beam radiation, the rectal dose, and tobacco consumption. The external beam data is beginning to show that long-term hormonal therapy (nine months or more) causes more rectal bleeding following external beam radiation. We evaluated that but did not find that hormonal therapy had any effect on rectal function following brachytherapy.
Sexual function following brachytherapy
Erectile function is one of the most overestimated outcomes. The Internet and individual publications report that brachytherapy results in potency preservation in 80 percent of patients. We did not believe those numbers, and we published the first data using a validated instrument, the International Index of Erectile Function (IIEF).
Using the IIEF-5, our six-year potency preservation rate after brachytherapy was 39 percent. The Cleveland Clinic data, using the IIEF-6, found only 19 percent of patients maintained potency after brachytherapy. Our potency rates also fall to 25 percent if we use comparable scoring. Fortunately, sildenafil is very effective for these gentlemen. In our series, the use of sildenafil increased the six-year potency preservation rate to about 90 percent.
Postbrachytherapy PSA spikes
Prostate-specific antigen (PSA) spikes - usually defined as increases in PSA greater than or equal to 0.2 ng/mL followed by a durable decline - occur in 23 percent of our patients. In our series, most PSA spikes occurred between 12 and 30 months after an implant; however, they may still occur as much as five years later. We believe PSA spikes are a result of compromised cell-membrane integrity - a radiation-induced prostatitis.
Younger men are more likely to experience a PSA spike, possibly because they're sexually active. The first postimplant PSA level is a strong predictor of PSA spike. In our series, the first postimplant PSA level in men with PSA spikes was 1.2 ng/mL compared to 0.6 ng/mL in men without a PSA spike. Also, patients treated with an I 125 implant were twice as likely to have a PSA spike as those treated with Pd 103 implant (33 percent versus 17 percent, respectively).
It is important that patients, radiation oncologists, urologists and primary care physicians be aware of this PSA spike phenomenon so that we do not rush into a salvage prostatectomy or hormonal therapy in these men.
Kent Wallner and I recently published a report in Urology on a series of eight patients who had PSA spikes and biopsies that were positive for prostate cancer. It was recommended that all eight patients undergo salvage prostatectomy, but nothing was done and all the men had a subsequent decrease and normalization in their PSA level. Therefore, biopsies, at least in those first couple of years, are probably of limited utility in determining subsequent treatment.

Case discussion: 52-year-old man with a postimplant PSA spike that resolved without intervention
Approximately eight years ago, we had a patient with a PSA of 4.6 ng/mL and a Gleason 6 prostate cancer whom we treated with an I 125 implant. At that time, he was about 52 years of age. He had a poor initial PSA response with a PSA nadir of about 2.5 ng/mL approximately 18 to 24 months after the implant. Three and a half to four years after the implant, his PSA began to rise and went up to approximately 5.2 ng/mL. I was confident that he had failed. I did not recommend any additional intervention at that time and continued to watch him. He was very comfortable with this approach. His PSA went down to 4.8 ng/mL, and six months later, it went to about 0.3 ng/mL. Eight years after his initial implant, his PSA is zero. This is a unique case, and we don't usually see patients who are cured when the initial PSA nadir is that high. This patient was not overly concerned with his PSA. Fortunately, we did nothing and he's been fine.
Select publications
|