 Home: PCU 6|2003: Judd W Moul, MD, FACS Home: PCU 6|2003: Judd W Moul, MD, FACS
 |
|
Judd W Moul, MD, FACS |
| |
| |
Director, Department of Defense Center for Prostate
Disease Research
Professor of Surgery, Uniformed Services University
Attending Urologic Oncologist
Walter Reed Army Medical Center
Washington, DC |
Edited comments by Dr Moul
Intergroup S9921 adjuvant trial of hormonal therapy with or without chemotherapy
We have not yet proven whether adding adjuvant chemotherapy to hormonal therapy makes a difference. Intergroup trial SWOG-S9921 is enrolling patients who have undergone a radical prostatectomy and whose tumors have adverse pathologic features.
This trial evaluates whether the combination of hormonal therapy plus chemotherapy is better than hormonal therapy alone. We desperately need to complete this trial, but it's accruing slowly. After completing this study, we need to evaluate some of the potentially more effective chemotherapies along with hormonal therapy in the adjuvant setting.

Nonprotocol options for patients with high-risk disease after prostatectomy
In a nonprotocol setting, the standard approach to patients with high-risk prostate cancer has been close monitoring. In light of the Early Prostate Cancer trials with bicalutamide 150 mg monotherapy, some clinicians utilize this approach. Others put these patients on traditional hormonal therapy, such as LHRH agents plus or minus an antiandrogen, even though it's not considered the standard yet.
Many patients undergoing radical prostatectomy are younger and have a successful nerve-sparing procedure. Many of these men have regained or are regaining sexual function. The Intergroup trial has a control arm of traditional hormonal therapy, which has a profound effect on their sexual function. Some men who don't enroll on the Intergroup trial are opting for bicalutamide monotherapy as opposed to the traditional hormonal therapy.
RTOG Trial 85-31: Immediate versus delayed hormonal therapy in patients with locally advanced disease
RTOG-85-31 randomly assigned patients with locally advanced prostate cancer who were going to receive external beam radiotherapy to radiotherapy alone versus radiotherapy plus immediate traditional hormonal therapy.
The data presented from RTOG-85-31 at ASCO 2003 demonstrated an overall survival advantage for patients who received immediate versus delayed hormonal therapy. These findings confirm the results of the Bolla trial.
Many people criticized the Bolla trial because it was a single study and patients in the delayed treatment arm probably were treated later than in North America, magnifying the difference between early and the delayed treatment. RTOG-85-31 is more representative of North American practice patterns and demonstrates a survival benefit to androgen ablation.
Prostate Cancer Prevention Trial (PCPT): Interpreting the results
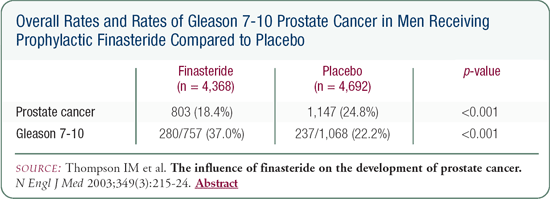
The Prostate Cancer Prevention Trial was designed to determine whether finasteride prevents prostate cancer. The trial enrolled over 18,000 men from across the United States. It had a very simple design that randomly assigned men 55 years of age or older - all of whom had a PSA of 3 ng/mL or less -to finasteride or placebo. The plan was for a seven-year follow-up. Any man who developed an abnormal digital rectal exam or a PSA that rose significantly underwent a prostate biopsy. The men who maintained a normal PSA or who did not develop a change in their digital rectal exam were asked to undergo a prostate biopsy at the end of seven years.
Men who received finasteride, compared to those who received placebo, had a 24.8 percent reduction in the "period" prevalence of prostate cancer -patients who developed prostate cancer during the seven years plus patients who were diagnosed with prostate cancer at the end of seven years. The study was stopped because the primary endpoint was achieved and the safety monitoring committee believed it would be unethical to continue the study because the reduction in prostate cancer had been met.
The bad news was that tumors in men who developed prostate cancer on finasteride had a higher rate of Gleason scores of seven, eight, nine or 10 compared to those in the men on placebo. When I explain these results to patients, I say, "If you take this drug, your chance of developing prostate cancer appears to be less, but if you develop prostate cancer, the cancer may appear more aggressive under the microscope."
Urologic pathology involves considerable debate. One school of thought contends finasteride actually changes the appearance of the cancer, making the Gleason scoring more difficult. The other school of thought says the change in Gleason score is a real biological phenomenon. Is it an epiphenomenon - the drug just changes appearances under the microscope - or a real phenomenon in which the drug actually increases the aggressiveness of prostate cancer? We don't know.
Case discussion: 67-year-old man on testosterone replacement with abnormal prostate biopsy
Treated for hypogonadism since 1995, this man had five sets of prostate biopsies between 1995 and 2002 with a reference diagnosis of probable cancer, not yet definitive. In February 2002, he was taken off of testosterone replacement therapy. The problem is that he's becoming more symptomatic from hypogonadism. His current testosterone level is 30 ng/dL - significantly lower than the normal level of greater than 250 ng/dL. The question is: How do we treat this man?
He has a few cells on one of five biopsies that have been interpreted as "prostate cancer," yet he doesn't want anything definitive done for this condition. He's in excellent health, and his attitude is that "life goes on." We've been equivocating as to whether or not he has cancer. He's probably had about 60 cores removed from his prostate over the last six years. One or two cores show a few isolated cells suggestive of cancer.
He's miserable because he has gained weight and has poor muscle tone, mood swings and no libido. He wants to go back on testosterone replacement therapy and says he'll shop around until he finds a physician who will restart therapy.
This issue may become more common in light of discussions of "andropause" among the aging male population. Is it truly wrong to maintain a normal testosterone in some of these men? The conventional wisdom is that prostate cancer is fueled by testosterone, and testosterone replacement should not be given. The reality is that more recent studies suggest men with low testosterone levels may have more advanced prostate cancer.
One recommendation would be to tell this patient, "Unless you have your prostate removed, you should not go on testosterone replacement therapy." Is that too Draconian for this man? I don't know the answer, but it's a real dilemma, including from a medicolegal perspective.
Prostatectomy versus radiotherapy for men with lower-risk disease
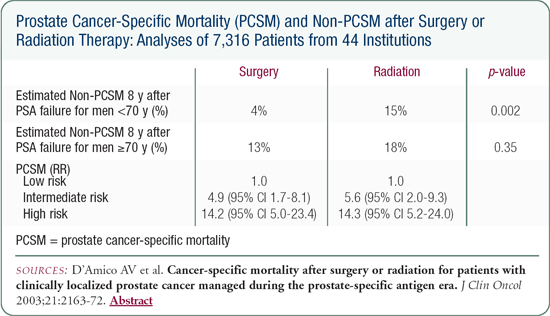
I recently had the fortunate opportunity to collaborate with Anthony D'Amico and Peter Carroll to combine our CPDR database with the CaPSURE database. We were able to amass over 6,000 patient records, asking the simple question of whether stratifying patients by the D'Amico risk stratification schema predicts death from prostate cancer. We found that in patients who underwent either surgery or radiation, this schema predicted death from prostate cancer at 10 years. Stratified by age, men with low-risk disease - PSA of 10 or less, Gleason score of 6 or less, T1C, T2A - who underwent surgery had a lower chance of death from prostate cancer than those who underwent primary radiation therapy.
The caveats are that this is not a randomized trial and the radiotherapy was predominantly conventional external beam radiation. Some would argue that with higher doses of radiation - intensity modulated or brachytherapy - it is reasonable to offer radiotherapy to younger men with low-risk disease.
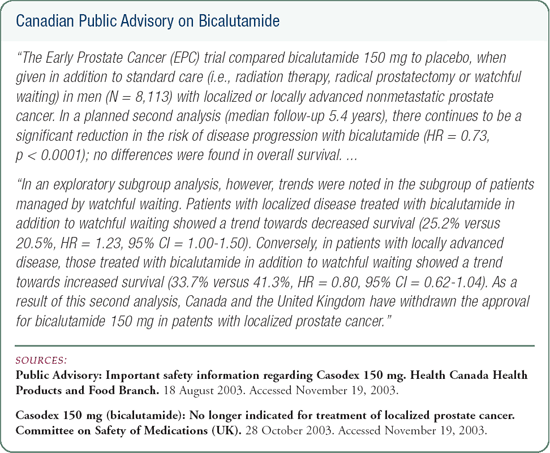
Use of bicalutamide 150 mg monotherapy in clinical practice
In the EPC trials, bicalutamide 150 mg monotherapy resulted in a clinical progression benefit. When it was published by Dr See in the Journal of Urology, there was a greater magnitude of benefit in patients with high-risk disease, and there was a lot of skepticism. Even though there was statistical benefit, the clinical benefit in individuals at low risk was not great.
Another concern is that compared to placebo, bicalutamide delayed the onset of clinical metastases, but there was no survival benefit observed.
Additionally, in the North American trial made up of mostly radical prostatectomy patients, many men had organ-confined prostate cancer and would be considered at low risk for progression.
These patients, who were probably included in the low-risk group, may have diluted the overall effect. The bottom line is immediate adjuvant bicalutamide 150 mg makes sense for patients who are risk-stratified and may be at higher risk for progression.
The EPC trial did not evaluate therapy at PSA progression. It compared men who were treated adjuvantly to men who underwent watchful waiting, external beam radiation or radical prostatectomy. It's speculative to extrapolate the use of bicalutamide to biochemical recurrence. Many physicians use it in that setting and assume the benefits will be the same, but we have to be academically honest: The trial did not look at biochemical recurrence.
The use of bicalutamide 150 mg has increased in younger men who experience a biochemical recurrence, or men who fall into the very high-risk postoperative group. This is particularly true for men who are recovering sexual potency after a successful nerve-sparing prostatectomy.
Those men are reluctant to use traditional hormonal therapy because they know it will hinder their sexual rehabilitation and further recovery of their erectile function. They've been more willing to take bicalutamide monotherapy, hoping it will have less impact on their sexual function. The problem is that we don't have long-term data to know whether sexual function is truly maintained. We believe it will be, but we don't have data on men in their forties and fifties who have been through a nerve-sparing procedure.
Tolerability of bicalutamide monotherapy
In the short-term, breast irradiation seems to eliminate the breast enlargement and most of the tenderness associated with bicalutamide. Many patients will still have mild nipple tenderness without a great deal of breast growth.
Patients receiving bicalutamide alone do not develop hot flashes. I've carefully questioned patients on bicalutamide who have been through a nerve-sparing prostatectomy about their experiences, and they tell me their sexual libido is maintained and they still respond to sildenafil. Men who were regaining natural potency continue to do so. I have to admit, I have fairly short follow-up and I have not used an objective questionnaire to systematically evaluate sexual function in these patients.
Select publications
|